Human papillomavirus (HPV) is the most prevalent sexually transmitted infection. It is more common than herpes and chlamydia and it has been estimated that more than 75% of sexually active adolescents and adults between the ages of 15 and 49 will acquire at least one type of HPV infection. The infection is practically guaranteed in people with more than five sex partners in their lifetime.
More than 100 different HPV genotypes exist, approximately 10 to 15 of which are known to cause disease in genital mucosal tissues. These include nononcogenic varieties—primarily types 6 and 11, as well as types 42, 43, and 44—associated with the development of genital condylomas or mild dysplasia and generally do not progress to either higher-grade lesions or cancer. Oncogenic varieties—types 16 and 18, in particular—are associated with more dysplastic lesions and account for the majority of invasive cervical or anal carcinoma cases. Types 31, 33, and 35 are associated with an intermediate risk of high-grade dysplasia and cancer.
| Incidence of Anal Cancer | Top of page |
Anal cancer is fairly rare. Its incidence in the general population is less than one per 100,000 people and is one-tenth the current rate of cervical cancer in the United States. However, more startling numbers come in to play when evaluating the incidence of anal cancer among specific populations. In a 1987 paper published in the New England Journal of Medicine, it was estimated that the incidence of anal cancer among HIV-negative men who engage in receptive anal intercourse with other men was up to 35/100,000—a rate on a par with the incidence of cervical cancer before routine Pap smears were initiated in the 1940s (Daling, 1987).
Even more alarming is the incidence rate among HIV-positive men who have sex with men (MSM). During the late 1980s, the incidence of anal cancer among gay men with AIDS was reported to be twice that of men of the same age, race, and sexual orientation in the years before AIDS (1975 to 1979) (Goedert, 1998). In other words, the incidence of anal cancer may be greater than 70 of every 100,000 HIV-infected MSM who engage in receptive anal intercourse.
Newer data suggest the incidence of anal cancer is increasing in the United States. In one study, age-adjusted incidence rates were calculated by gender, race/ethnicity, county, and year of diagnosis for over 2,100 cases of anal cancer in California between 1995 and 1999 (Cress, 2003). Age-adjusted incidence rates in San Francisco County, from 1973 to 1999, were also evaluated.
Generally speaking, the incidence rates of anal cancer were higher for women than for men in California. However, men under 40 years of age and those classified as black had higher age-adjusted rates than women. Men in San Francisco County also had higher anal cancer rates than women. For all of California, there was an average 2% annual increase among Caucasian men between 1988 and 1999. The incidence of anal cancer among Caucasian males residing in San Francisco County more than doubled between the 1984 to 1990 and 1996 to 1999 time periods. The increasing incidence rates were most dramatic in San Francisco men between the ages of 40 to 64, from 3.7 cases per 100,000 in 1973 to 1978 to 8.6 cases per 100,000 in 1984 to 1990, and to 20.6 cases per 100,000 in 1996 to 1999.
“Unfortunately,” Dr. Goldstone commented, “these data do not address incidence rates among gay men or among HIV-positive individuals.” In turn, Dr. Goldstone highlighted new data from Dr. Catherine Diamond and her colleagues, using linked data from cancer and AIDS registries in San Diego County (Diamond, 2005).
Dr. Diamond’s group compared the annual incidence of anal cancer among HIV-infected men in the five-year periods before and after the introduction of potent combination antiretroviral therapy (1991 to 1995 vs. 1996 to 2000). They identified 2,055 men who had both HIV-infection and cancer; 42 had anal cancer specifically. Of these 42 men, 37 were diagnosed with HIV before or at the same time that they were diagnosed with anal cancer. The median duration of HIV infection prior to anal cancer diagnosis was significantly longer for men diagnosed after the introduction of potent combination antiretroviral therapy than for men diagnosed before (84 vs. 22 months). Pathology revealed in situ disease in 12 men and invasive disease in 30; cancer stage did not differ significantly by time period of diagnosis.
More cases of anal cancer were diagnosed after the introduction of potent combination antiretroviral therapy than before (33 in 1996–2000 vs. 9 in 1991–1995). The annual incidence of anal cancer among HIV-positive men aged 25 to 64 increased significantly after the introduction of potent combination therapy (from 88 per 100,000 in 1991–1995 to 190 per 100,000 in 1996–2000). Anal cancer incidence did not increase significantly among men without known HIV infection.
| Natural History of HPV Infection | Top of page |
Approximately 80% of women infected with HPV are able to clear the infection within a year. “We don’t really have great natural history data in men who have sex with men,” Dr. Goldstone said. “We also don’t have a lot of natural history data with respect to women and anal disease.”
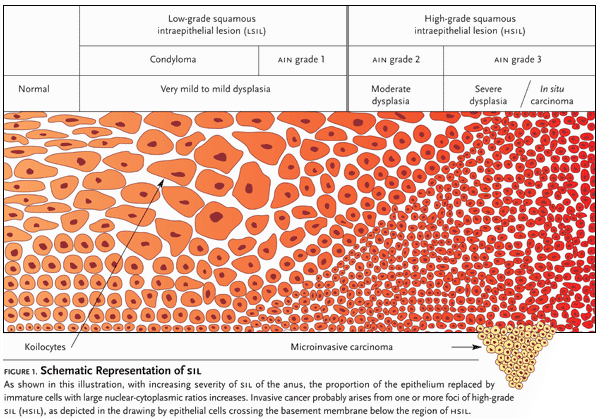
Figure 1. Schematic Representation of SIL
A fundamental goal of New York City Department of Health and Mental Hygiene HIV/AIDS programming is to curb the epidemic. However, there are significant challenges ahead, including the well-documented resurgence of risky sexual behavior. As is shown here, syphilis cases in New York City have increased more than four-fold over the past three years. The number of syphilis cases doubled between 2000 and 2001, increased another 46% in 2002, and another 22% in 2003. The increase is almost entirely among men, especially MSM.
Source: Joel Palefsky, MD, FCRP(C)
According to research published in 1998, a number of risk factors for HPV infection persistence—at least in women—have been identified (Ho, 1998). The presence of high-risk genotypes, such as HPV-16 and HPV-18, are of central concern. The presence of multiple subtypes is also an issue. “This is important because most men who have sex with men have a smorgasbord of HPV types in their anal canals,” Dr. Goldstone pointed out. “It’s rare that we get only one type of HPV. Usually we get two, three, or four.” Smoking in women has also been shown to correlate with increased persistence of HPV, as has older age at the onset of infection.
HPV infection initially takes place at the basal cell layer of the anogenital epithelium. In the anal canal, HPV infection often occurs in the transformation zone, located at the junction of the stratified squamous epithelium of the anus with the columnar epithelium of the rectum. The squamous cells in this area are often highly reactive and susceptible to viral infection.
Several classification systems have been used for the purpose of grading the cellular atypia seen in HPV lesions, varying from relatively benign lesions to invasive malignant neoplasms. The Bethesda system is now the accepted standard for classifying and staging cytology for anal precancerous lesions (ASC, 2003).
At one end of the HPV disease spectrum are anal warts (condyloma acuminata) and mild dysplasia—anal intraepithelial neoplasia grade 1 (AIN I)—also referred to as low-grade squamous intraepithelial lesions (LSIL). At the other end of the spectrum are AIN grades 2 and 3—also known as moderate and severe dysplasia, respectively—and carcinoma in situ. The Bethesda system categorizes these changes as high-grade squamous intraepithelial lesions (HSIL). “The reason why we have two groupings of either low-grade or high-grade dysplasia,” Dr. Goldstone pointed out, “is because of wide discrepancy between AIN 2 and AIN 3. There isn’t good correlation between different pathologists.”
| Incidence of HPV and AIN | Top of page |
Data regarding the incidence of anal HPV infection have been reported. In one study published in 1998, Dr. Joel Palefsky and his colleagues at the University of California, San Francisco, looked for anal HPV infection in 346 HIV-positive and 262 HIV-negative MSM (Palefsky, 1998). Anal HPV-DNA was found in 93% of the HIV-positive men, compared with 61% of the HIV-negative men.
HPV-16 was the most common type present in both HIV-positive and HIV-negative MSM. Infection with multiple HPV types was found in 73% of the HIV-positive men and 23% of the HIV-negative men.
Also of interest was an association between the presence of oncogenic HPV types and CD4+ cell counts. Nononcogenic HPV types (e.g., HPV-6; HPV-11) were documented with equal frequency in HIV-positive patients with less than 200 CD4+ cells/mm3, between 200 and 500 cells/mm3, or greater than 500 CD4+ cells/mm3. Oncogenic HPV types (e.g., HPV-16; HPV-18) were more common in patients with CD4+ counts below 200 cells/mm3 than in patients with greater than 500 CD4+ cells/mm3, a finding that was indicative of immune suppression being a significant cofactor in the replication of oncogenic, HSIL-causing HPV types.
As for the natural history of AIN in gay and bisexual men, prospective studies conducted in Seattle and San Francisco have yielded interesting results. In the Seattle cohort, 158 HIV-positive and 147 HIV-negative MSM without initial evidence of anal squamous intraepithelial lesions were monitored for an average of 21 months (Critchlow, 1995). In less than two years, HSIL developed in 24/158 (15%) of the HIV-positive men and 8/147 (5%) of the HIV-negative men. In the San Francisco cohort, 277 HIV-positive MSM and 221 HIV-negative MSM—all of whom entered the study with either normal anal Pap test results or LSIL—were followed prospectively for approximately four years (Palefsky, 1998a). During this period, 49% of the HIV-infected men developed HSIL, compared with 17% of the HIV-negative men. What's more, HIV-positive men with either no or low-grade dysplasia at baseline were more likely to develop HSIL during the four years of follow-up, compared with HIV-positive men with normal anal Paps upon entering the study (57% vs. 38%, respectively). As for HIV-negative men with no dysplasia or LSIL at baseline, 33% developed HSIL during the four years of follow-up, compared with 14% of HIV-negative men who entered the study with normal anal cytology.
Despite the abundance of epidemiological data showing increased incidence and prevalence rates of HPV infection, anal dysplasia, anal cancer, and associations between the three, a central question still remains: does anal HSIL progress to cancer? “It’s not proven but the evidence is growing,” Dr. Goldstone commented. “Dr. Palefsky and I have approximately 45 patients with high-grade dysplasia who have progressed to cancer in the absence of treatment for HSIL.” He remarked that it takes many years—“probably between ten and twenty years”—for a high-grade lesion to develop into cancer. “Most high-grade lesions will never become cancer, much like we see in the cervix. It’s just that we don’t know which lesions will and which lesions won’t develop into anal cancer. You would need a huge sample size with long-term follow-up to look at it.”
Dr. Goldstone also discussed the example of Bowen’s disease, a high-grade lesion—or carcinoma in situ—with the potential for significant lateral spread. “It can affect the perianal area,” Dr. Goldsone commented, “and it is often caused by HPV infection. It is histologically indistinguishable from other high-grade lesions in the anal canal. Even though it is only a precancerous lesion, surgeons routinely believe that it should be treated, yet the same surgeons question the need to treat high-grade anal lesions.”
| Screening for Anal Dysplasia | Top of page |
There is no denying that cervical Pap smear and colposcope screenings have had a profound effect on the incidence of cervical cancer, among both HIV-positive and HIV-negative women. If we are to assume that anal dysplasia is similar to cervical dysplasia in its natural history and pathogenesis, compounded by the seemingly high prevalence and incidence of HSIL in certain populations, the suggestion that anal cytology screenings may play an invaluable role in detecting high-grade dysplastic lesions is merited.
Dr. Goldstone and numerous other experts recommend that all MSM undergo regular anal cytology, regardless of HIV infection status. Other groups who should be considered for screening include: women with cervical cancer or high-grade vulvar disease/cancer; all HIV-positive men and women, regardless of sexual orientation; individuals with perianal condyloma acuminata; and other immune-compromised individuals such as transplant recipients.
Obtaining a specimen for anal cytology is a simple procedure. First, a Dacron swab is inserted approximately 1.5 to 2 inches into the anal canal. Dr. Goldstone indicated that it’s necessary to use a Dacron swab, not a cotton swab, as the cells cling to the cotton and won't be released easily for cytology. “Laboratories conducting anal cytology will gladly provide Dacron swabs,” he noted. Dr. Goldstone also stressed that lubricant should not be used. “Lubricant can result in an inadequate sample. Prior to inserting, the swab should be dipped in water to provide some comfort to the patient without ruining the sample. This also means that the anal cytology should be performed before the digital rectal exam, which requires lubricant.”
Once inserted deep enough into the anus—this is necessary, in order to collect both rectal columnar and anal squamous cells—the swab should be moved in and out several times, applying some pressure to the wall of the anus, rotating the swab in a spiral motion along the way. From there, the cells collected with the swab can be placed in a fixative medium or spread onto a glass slide and sprayed with fixative solution and, finally, shipped off to a cytology lab for analysis. “It is sent to the laboratory, not as a Pap smear, but as anal cytology,” Dr. Goldstone added. “There’s a special code for it and the pathologists can all read it.”
A digital rectal exam is performed after the Pap has been completed. The rectal exam begins with an external inspection. A lubricated finger is then inserted into the anus to palpate the anal wall. “You go all the way in until you feel the prostate, but then you need to withdraw. You want to feel the anal ring, which is the most distal, one to two centimeters in the anal canal. That’s where you’re going to feel HPV-related disease. If you’re way up, you’re above the squamocolumnar junction, where there won’t be HPV disease.”
Dr. Goldstone also recommends basic anoscopy—a short, lighted tube used to visually inspect the anus—for all patients who engage in receptive anal sex as a component of routine anorectal exams.
In the event of abnormal Pap findings—whether it be ASCUS, LSIL, or HSIL—high-resolution anoscopy should be performed. To visualize the anal wall, a disposable anoscope is used. It is inserted through the anal canal using a water-soluble lubricant; topical anesthetic can also be used. A swab, wrapped in gauze and soaked in 3% acetic acid, is passed through the anoscope. The anoscope is then removed, leaving the acetic acid-soaked swab in the anus, pressed up against the anal epithelia, for approximately one minute. The swab is then removed and the anoscope reinserted. A colposcope is then used to visualize the anal canal and distal rectum as seen through the anoscope. “You really need to magnify the tissue because you’re looking for subtle changes,” Dr. Goldstone said.
Whereas healthy epithelial tissue inside the anus is pink and shiny, the application of acetic acid will turn any inflamed tissue, including dysplasia, dull and white. Acetowhite areas should be inspected for changes indicative of dysplasia such as punctuation and mosaicism. If areas suspicious for HSIL or even cancer are seen, a biopsy should be taken. Another dye that can be used is the iodine-based Lugol's solution. When taken up by healthy epithelial tissue, it will render the anal wall a deep mahogany color. Conversely, dysplastic lesions do not fully absorb this iodine-rich solution and will likely turn a mustard or light yellow color. These, too, should be examined and biopsied for definitive diagnosis.
In June 2000, Dr. Goldstone presented important data to the American Society of Colon and Rectal Surgeons (Goldstone, 2000). These data came from Dr. Goldstone’s surgical practice in New York and included 200 men referred to him for care. Of the 200 men, 131 were HIV-positive and 69 were HIV-negative. The median age was 36 years. One-hundred fifty-seven (78%) men were referred to Dr. Goldstone for condyloma, 39 (20%) were referred to him for non-condylomatous disease (e.g., hemorrhoids, fissures, etc.), and four (2%) men were referred for treatment of HSIL or LSIL.
Dr. Goldstone performed anal Pap smears on all 200 men. The cytology results were starkly different from the reasons for referral: Four (3%) of the HIV-positive had benign cytology results, 10 (8%) had ASCUS, 42 (32%) had LSIL, and 75 (57%) had HSIL. Among the HIV-negative men, 10 (14%) had benign cytology results, 12 (17%) had ASCUS, 17 (25%) had LSIL, and 30 (43%) had HSIL. “Only four of the men were referred to us for LSIL or HSIL,” Dr. Goldstone said. “But when we conducted cytology screenings, it was clear that we were dealing with a total of 59 patients with LSIL and 105 patients with HSIL.”
Biopsy results from the pathology lab painted an even more startling picture. Among the HIV-positive patients, three (2.3%) had benign biopsies, two (1.5%) had condyloma, 33 (25.2%) had LSIL, 89 (67.9%) had HSIL, and 4 (3.1%) had invasive anal cancer. Among the HIV-negative patients, eight (11.6%) had benign biopsies, 11 (14.9%) had condyloma, 18 (26.1%) had LSIL, 31 (44.9%) had HSIL, and one (1.4%) had invasive anal cancer. “Almost three-quarters of our HIV-positive patients had either HSIL or anal cancer,” Dr. Goldstone said.
Dr. Goldstone also reviewed the correlations between the cytology results and the pathology results. Of the patients who had benign cytology results, eight (57.1%) had benign biopsies, three (21.4%) had condyloma, one (7.1%) had LSIL, one (7.1%) had HSIL, and one (7.1%) had invasive cancer. Among patients who had Pap smears yielding ASCUS, two (9.1%) had benign biopsies, four (18.2%) had condyloma, five (22.7%) had LSIL, 11 (50%) had HSIL, and none had cancer. As for patients with Pap smears that yielded LSIL, one (1.8%) had benign biopsies, three (5.3%) had condyloma, 26 (45.6%) had LSIL, 24 (42.1%) had HSIL, and three (5.3%) had invasive cancer. Finally, among patients with Pap smears yielding HSIL, zero had benign biopsies, three (2.8%) had condyloma, 19 (17.8%) had LSIL, 84 (78.5%) had HSIL, and one (0.9%) had cancer. “Any abnormal cytology correlated with a 66% incidence of high-grade dysplasia or cancer,” Dr. Goldstone calculated. “This is very similar for the cervix. Any low-grade or high-grade cytology correlated with a 68% incidence of HSIL or cancer. Most concerning, of course, is the fact that a percentage of our patients had invasive anal cancer, most of whom were referred for non-condyloma disease. This definitely speaks to the importance of cytology and pathology. Cytology must not be viewed as providing a definitive diagnosis. At best it is a predictor of disease.”
| Treatment of Anal Cancer | Top of page |
Anal cancer is typically staged using a classification system set up by the National Cancer Institute. The NCI staging system for anal cancer is described in Table 1.
| Table 1. The NCI Staging System for Anal Cancer | ||||||||||||
| Stage | Qualification | |||||||||||
| 0 | Carcinoma in situ, intraepithelial carcinoma. Corresponds to the following TNM groupings: Tis, N0, M0 |
|||||||||||
| I | Cancer that is 2 centimeters or less in greatest dimension and not spread anywhere else. There is no sphincter involvement. Corresponds to the following TNM groupings: T1, N0, M0 |
|||||||||||
| II | Cancer that is more than 2 centimeters and does not involve adjacent organs or lymph nodes. Corresponds to the following TNM groupings: T2, N0, M0 T3, N0, M0 |
|||||||||||
| IIIA | Cancer that has spread to perirectal lymph nodes or to adjacent organs. Corresponds to the following TNM groupings: T1, N1, M0 T2, N1, M0 T3, N1, M0 T4, N0, M0 |
|||||||||||
| IIIB | Cancer that has spread to internal iliac and/or inguinal nodes (unilateral or bilateral) or has spread to both adjacent organs and perirectal lymph nodes. Corresponds to the following TNM groupings: T4, N1, M0 Any T, N2, M0 Any T, N3, M0 |
|||||||||||
| IV | Cancer that has spread to distant lymph nodes within the abdomen or to other organs in the body. Corresponds to the following TNM groupings: Any T, Any N, M1 |
|||||||||||
|
||||||||||||
Standard treatment of anal cancer involves chemotherapy combined with radiation, sometimes referred to as chemoradiotherapy. Fluorouracil or mitomycin C is usually administered intravenously for a week, followed by 45 Gy localized radiation therapy (usually over a period of five weeks), and completed with another week of chemotherapy.
Unfortunately, there are data suggesting that HIV-positive patients with anal cancer have poorer treatment tolerance and outcomes than HIV-negative patients. In one published series, clinicians associated with the Division of Colon and Rectal Surgery at George Washington University Medical School reviewed their experience with anal carcinoma and compared HIV-positive to HIV-negative patients by age, gender, sexual orientation, stage at diagnosis, treatment rendered, response to treatment, tolerance, and survival (Kim, 2001).
From 1985 to 1998, 98 patients with anal neoplasms were treated. Seventy-three patients had invasive squamous-cell carcinoma (including cloacogenic carcinoma), and this cohort was analyzed. Thirteen patients were HIV positive and 60 were HIV negative.
The HIV-positive and HIV-negative groups differed significantly by age (42 vs. 62 years respectively), male gender (92% vs. 42% respectively), and homosexuality (46% vs. 15% respectively). There were no differences by stage at diagnosis or radiation dose received. Major toxicity was significantly more likely to occur in the HIV-positive patients than in the HIV-negative patients (80% vs. 30% respectively). Only 62% of HIV-positive patients were rendered free of disease after initial therapy, compared to 85% of the HIV-negative patients. However, this difference was not statistically significant. Median time to cancer-related death was 1.4 vs. 5.3 years, a statistically significant difference in favor of the HIV-negative patients. A survival model did not show age, gender, stage, or treatment to be independent predictors. “These data are distressing,” Dr. Goldstone noted. “They definitely underscore the need for early and prompt diagnosis of pre-cancerous lesions, especially in HIV-infected individuals.”
| Treatment of HSIL | Top of page |
Treatment of HSIL of the cervix generally involves removal of the squamocolumnar transition zone. “This can’t be done in the anus,” Dr. Goldstone said. “However, if a lesion can be identified in the anus, it can be treated.”
High-resolution anoscopy-directed surgical removal is the typical treatment for HSIL. Of interest are the results of a prospective study of HSIL excision/cauterization performed in an operating room in 37 men, 29 of whom were HIV-positive and eight of whom were HIV-negative (Chang, 2002). While none of the eight HIV-negative study participants developed recurrent HSIL, 23/29 (79%) of the HIV-positive patients had persistent or recurrent HSIL, usually within a year of surgical removal. Six patients underwent a second surgery for HSIL; four patients had persistent or recurrent disease within six months. No patients developed incontinence, stenosis, postoperative infection, or significant bleeding after surgical treatment. “However,” Dr. Goldstone noted, “half reported uncontrolled pain lasting for a mean of 2.9 weeks. We all know this. It hurts like hell when you have to take patients to surgery for extensive disease.”
One of Dr. Goldstone’s primary interests has been the evaluation of office-based methods to treat HSIL. “If it’s a mucosal lesion, why can’t we ablate the dysplasia without doing radical surgery?” Dr. Goldstone asked. Options used by Dr. Goldstone include liquid nitrogen, 80% trichloroacetic acid, surgical excision, and laser ablation, all of which produce encouraging results. “However, thick lesions are much more difficult to ablate. Lesions can be above the dentate line, so it’s difficult for us to reach them with standard techniques. And with topical agents or liquid nitrogen, we don’t know how deep the destruction is; we don’t know if we’re getting through the lesion. In turn, patients need repeated follow-ups.” This, Dr. Goldstone explained, is burdensome for the patients and burdensome for the practice.
“If anal intraepithelial neoplasia is the anal squamous-cell carcinoma precursor,” Dr. Goldstone said, “then by ablating HSIL we hopefully will prevent progression to cancer.” Ideally, he was looking for a procedure that was office-based, well tolerated with minimal time out of work, afforded acceptable recurrence rates, and able to be performed by non-surgeons. “Treatment of HSIL doesn’t need to be performed by anal surgeons,” he added. “Options that can be handled by primary care providers (physicians or non-physicians) are needed.”
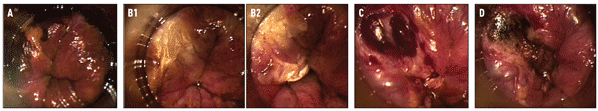
Figure 2. Infrared Coagulation: Procedure
A. Shown here in the upper left-hand corner is a well circumscribed high-grade anal squamous intraepithelial lesion (HSIL) that appears must-colored after Lugol's staining of the surrounding squamous epithelium turns normal tissue and low-grade SILs black.
B. Blunt dissection was used to separate the eschar after successful desiccation of the HSIL. The base can then be debrided with sharp dissection to ensure destruction of the full thickness of the lesion.
C. A bulging vein is visible after the lesion has been systematically coagulated and debrided to a depth of the mucosal vessels.
D. The coagulated submucosal vessel with a dark thrombus is visible. The lesion has been successfully ablated through its entire thickness.
Figure Grouping Stephen E. Goldstone, MD, FACS.
In 1999, Dr. Goldstone began using the IRC2100 Infrared Coagulator, manufactured by the Redfield Corporation in Rochelle Park, NJ, as an alternative surgical modality for the in-office treatment of anal dysplasia. The device uses a beam of far-infrared light delivered through a light guide covered with a disposable plastic sheath to ablate tissue and coagulate blood in the immediate surface area in contact with the tip. The IRC beam can be pulsed at 0.5- to 3.0-second intervals, thereby preventing trauma to deeper tissues. The depth of tissue coagulation is roughly equal in millimeters to the length of the pulse applied. In other words, a one-second pulse penetrates the tissue to a depth of approximately 1 mm. Pulse duration longer than three seconds produces char with burning of surrounding tissues. The surgical procedure using infrared coagulation is illustrated in Figure 2.
To evaluate its efficacy, Dr. Goldstone and his colleagues conducted a retrospective review of 68 HIV-positive MSM who have undergone infrared coagulation (IRC) in his office since 1999 (Goldstone, 2005). All patients had biopsy-proven HSIL and ablation was performed with local anesthesia. All patients had at least six months of follow-up. Follow-up consisted of anal cytology with high-resolution anoscopy and biopsy of suspicious areas every three to six months. New or recurrent high-grade dysplasia was retreated. Patients with circumferential or bulky disease were treated in the operating room and were excluded from the study.
A total of 165 lesions were treated (mean 1.6 lesions per patient). Forty-four (65%) patients developed new or persistent HSIL within a median time of 217 days after IRC. The remaining 24 (35%) patients were free of HSIL for a median of 413 days after IRC. The cure rate of any single lesion treated, however, was 72%. When patients were treated a second or third time, the incidence of new or persistent HSIL dropped to 58% and 40% respectively. The probability of curing a retreated lesion was 72%.
Using generalized estimating equations, Dr. Goldstone’s group determined that the incidence of HSIL decreased with repeated IRC treatments. “No patient developed infection, bleeding, or anal stricture,” Dr. Goldstone explained. “No patient required anything more than mild oral narcotic analgesia. Finally, and perhaps most importantly, no patient developed invasive squamous-cell carcinoma.”
In comparison with other studies, Dr. Goldstone suggested that his results using IRC are favorable. “In a small series utilizing electrocautery ablation of anal HSIL, there was a 79% recurrence rate over similar follow-up,” he said, referring to a study published by a team of surgeons at the University of California, San Francisco (Chang, 2002). “Gynecologic literature looking at treating CIN in HIV-positive women with LEEP [loop electrical excision procedure] demonstrates recurrence rates between 62% and 73%. When individual lesions in the cervix are treated by ablation rather than LEEP excision, recurrence rates can be as high as 90%. Our recurrence rate of 65% compares favorably with these series.”
In conclusion, Dr. Goldstone suggested that IRC is a safe office-based modality for treating anal HSIL in HIV-positive MSM. While recurrence rates are high, they are comparable to results seen in the cervix and in another surgical series. Successive treatments led to decreased recurrence rates, but patients should continue to be followed for disease. “An entire lesion can be treated in one sitting—or bending over—and results in fewer procedures and visits.”
| Conclusion | Top of page |
Dr. Goldstone wrapped up his lecture by discussing the elements of anal screening programs that should be incorporated into the care of all men who have sex with men. At the time of the initial screening, if the cytology is normal, it is recommended that an anal Pap smear be repeated annually for HIV-positive men, and every two to three years for HIV-negative men. If the cytology is abnormal—whether it be ASCUS, LSIL, or HSIL—the patient should be referred for high-resolution anoscopy. If no abnormality is seen, an anal Pap smear should be repeated in six months. If, however, an abnormal area is visualized, a biopsy should be performed under high-resolution anoscopy. A diagnosis of invasive anal cancer requires immediate treatment with appropriate protocol and follow-up. A benign biopsy or a finding of LSIL should be followed by a repeat Pap smear in six months. If the biopsy finding is HSIL, ablation or excision is recommended, followed by repeat high-resolution anoscopy in six months to rule out recurrence.
Dr. Goldstone acknowledged that anal cytology is not a routine component of HIV clinical care. “There is clinician disagreement about the utility of anal cytology,” he said. There’s a lack of trained personnel. This is a big problem, which is why I’m trying to empower clinicians to learn how to do this. For cervical colposcopy, clinicians need to perform 50 supervised cases before they’re certified to do it alone. You can’t do that with high-resolution anoscopy. There aren’t enough people to watch you do 50 cases. Skill sets are different based on prior experience. For instance, nurse practitioners and physician assistants may be able to pick up the techniques much quicker than doctors because they can be experienced in performing colposcopy or other surgical procedures. For clinicians, there’s a lack of time to perform these procedures. Plus, if you find HSIL, the clinician must be able to treat it, or at least have a referral to someone who can treat it. At the very least, all providers must perform frequent rectal examinations on HIV-positive MSM. Any abnormality must be investigated.”
Dr. Goldstone pointed out that the future of anal cytology looks bright. “This is consumer-driven,” he said. “Patients are learning about anal dysplasia and the risk of anal cancer and are asking their providers for Pap smears.” Unfortunately, it is also becoming liability-driven. “One patient we have with anal cancer is suing his doctor for failure to diagnose.”
More extensive training among healthcare providers, along with better treatments, will yield a greater desire to screen patients. Such improvements will also render diagnosis and treatment to be less painful and time-consuming to the patient. A win-win situation if there ever was one.
| References | Top of page |
