| Introduction | Top of page |
Since the advent of highly active antiretroviral therapy (HAART), the goals of treatment of HIV-infected patients have radically shifted. In addition to suppressing viral activity and maintaining immunocompetence, HIV treatment providers are also managing chronic complications of therapy and conditions related to aging. Endocrine and metabolic problems are often encountered among HIV-infected patients. This review focuses on 2 endocrine abnormalities, osteoporosis and adrenal insufficiency, summarizes the current evidence regarding the optimal evaluation of these disorders, and highlights their causes, presentation, and treatment in HIV-infected individuals.
| Osteoporosis | Top of page |
AG is a 62-year-old white male with a history of HIV disease, diagnosed in 1987 with a CD4 nadir of 22 cells per mm3. He is currently receiving an antiretroviral regimen composed of abacavir, zidovudine, lamivudine, and efavirenz (CD4 cell count: 476 cells/mm3, undetectable viral load[VL]). His ongoing HIV disease management has been complicated by peripheral lipoatrophy, central lipohypertrophy, and hypogonadism. He also has a history of chronic obstructive pulmonary disease and has received multiple courses of oral steroids in the past. He has no history of fracture, no height loss, and no history of heavy alcohol use. His daily calcium intake is estimated at 500 mg. Dual x-ray absorptiometry (DXA) revealed a lumbar spine bone mineral density (BMD) of 0.784 g per cm2 (T-score: -2.8, Z-score: -2.1), femoral neck BMD of 0.559 g per cm2 (T-score: -2.7, Z-score: -1.7), and total hip BMD of 0.637 g per cm2 (T-score: -2.6, Z-score: -2.1). Subsequent laboratory evaluation was significant for vitamin D deficiency (25-hydroxyvitamin D: 17 ng/mL; normal range: 20-100 ng/mL). Serum concentrations of parathyroid hormone, calcium, phosphorous, thyroid-stimulating hormone, and testosterone were all within normal limits. His vitamin D was replaced (ergocalciferol 50,000 units twice a week for 6 weeks) and he was started on risedronate 35 mg per week, in addition to 1000 mg per day of calcium supplementation.
| How is osteoporosis defined? | Top of page |
Osteoporosis is “a systemic skeletal disorder characterized by low bone mass and microarchitectural deterioration of bone tissue, with a consequent increase in bone fragility and fracture.”1 In clinical practice, low bone mass is generally measured by DXA. The World Health Organization (WHO) classifies BMD as normal, osteopenia, or osteoporosis according to the number of standard deviations by which BMD falls below the mean BMD for a healthy, young (aged 25-35 years), gender and ethnicity-matched reference population (T-score).2 Although this definition was created to evaluate BMD in postmenopausal women, it is generally applied to all adult populations, despite lack of evidence for its utility in men and in premenopausal women.
Osteoporosis is classified by DXA at the hip or the spine as a T-score lower than -2.5, whereas osteopenia is considered to be a T-score between -1.0 and -2.5. The Z-score is reported as the number of standard deviations by which BMD falls below the mean BMD for a healthy, age-, gender- and ethnicity-matched reference population, and can be useful in men and premenopausal women. Regardless of the BMD, osteoporosis can also be suggested by the presence of atraumatic fractures.
Osteomalacia (inadequate bone mineralization) can also be associated with a low BMD by DXA and is most often caused by vitamin D deficiency or phosphate wasting. This condition should be considered in any patient with low BMD.
| What are the risk factors for osteoporosis in HIV-infected patients? | Top of page |
In a recent meta-analysis of cross-sectional studies, we found that the prevalence of osteoporosis was more than 3 times more common in HIV-infected patients compared with HIV-negative controls.3 The cause of the higher risk of osteoporosis in HIV-infected patients is likely multifactorial, with possible contributions from antiretroviral therapy, HIV infection per se, and patient-related factors.
The initiation of antiretroviral therapy is associated with small, but significant reductions in bone mineral density. In a large randomized, double-blinded trial comparing the safety and efficacy of tenofovir to stavudine with a lamivudine and efavirenz backbone (Gilead 903), BMD decreased in both groups by approximately 2.5% over the 144-week study interval, with most of the decrease in the first 24 weeks.4 Similarly, in a recently presented substudy of ACTG 384,5 after initiation of antiretroviral therapy, the average reduction in bone mineral content was 2.25% over 96 weeks, with no differences based on the composition of the antiretroviral regimen received.
Previous studies have suggested that reduced BMD may be a direct toxic effect of antiretroviral medications. Protease inhibitors (PIs) were implicated in the prevalence of reduced BMD in one of the first studies of bone density in the HAART era,6 but other studies have failed to confirm this observation.7-9 However, patients who receive PIs may differ in other important ways from patients who receive PI-sparing regimens. These include the duration and severity of HIV infection, the duration of therapy, and the presence of comorbidities (eg, wasting) which may introduce selection bias into these comparisons. In addition, these opposing conclusions may be the result of heterogeneous effects of individual PIs on bone metabolism. In vitro studies have shown that indinavir exposure directly impairs bone formation, whereas ritonavir inhibits the osteoclast formation and therefore may be protective.10 In another in vitro study, saquinavir was shown to activate osteoclasts.11
Direct effects of nucleoside reverse transcriptase inhibitors (NRTIs) have also been implicated in the pathogenesis of reduced BMD in HIV-infected patients.12 Zidovudine (AZT) has been shown to stimulate osteoclast activity in vitro.13 Consistent with this observation, d4T or AZT exposure was associated with significant decrement in BMD over the 104 weeks,14 compared with those who were switched to abacavir. However, another “switch” study (TARHEEL) showed no changes in BMD after substitution of AZT or abacavir for d4T, despite significant improvements in peripheral fat.15
The nucleotide analogue, tenofovir, may have a greater effect on bone mineral density than the thymidine analog, d4T. In Gilead 903, there was a greater decline in BMD in those randomized to tenofovir compared with those receiving d4T at both the spine (–2.2% vs –1.0%, P=.001) and hip –2.8% vs –2.4%, P=.06).4 In certain patients, tenofovir impairs phosphate reabsorption in proximal tubule of the kidney16 and may also directly impair bone mineralization,17 thereby leading to osteomalacia.
Longitudinal studies of treatment-experienced patients with HIV have generally shown that BMD remains stable with continued antiretroviral exposure,18-21 providing evidence against a direct effect of antiretroviral therapy. The decrement in BMD with antiretroviral initiation, therefore, may be due to immunologic changes seen with antiretroviral initiation.5
In patients infected with HIV, the infection itself may also contribute to low BMD. Components of the virus, Vpr and gp120, have been shown to increase osteoclast activity, which leads to increased bone resorption.11,22
Factors independent of HIV or its treatment may also contribute to low BMD in HIV-infected patients. In several studies, low body weight has been shown to be an independent predictor of low bone mass9,23 and may represent the influence of confounding factors, such as severe HIV disease, malnutrition, wasting, immobility, and hypogonadism. Age is also another important risk factor,24 given that bone density decreases after early adulthood in all populations.25 Abnormal gonadal function in both men and women may also contribute to reduced BMD.26
Lifestyle factors, such as smoking, heavy alcohol use, and injection drug use have also been associated with reduced BMD.25,27,28 The extent to which these associations are confounded by other factors, such as poor nutrition (particularly dietary calcium and vitamin D deficiency), is unclear.
Concomitant medications, frequently used in HIV-infected patients, may also play a role. Glucocorticoids, whose effect may be augmented due to interactions with antiretrovirals (see below), decrease osteoblast activity and increase osteoclast activation.25 Other medications that may decrease BMD and/or increase the risk of fracture include depot forms of medroxyprogesterone, proton pump inhibitors,29 anticonvulsants, warfarin, and thiazolidinediones.30
| Are patients with HIV disease at a higher risk of fracture? | Top of page |
Given the higher prevalence of osteoporosis, it is suspected that the risk of fracture in HIV-infected patients is higher than in HIV-negative controls. In the general population, the risk of fracture in postmenopausal women increases by a factor of 1.5 to 3.0 for each standard deviation decrease in bone density below the young normal mean.31 The data in HIV-infected patients are limited. In a recent study of older men with or at risk for HIV infection,32 the risk of incident fracture was almost 3 times higher in those men with osteopenia and osteoporosis compared with those having normal BMD. Several case reports documenting atraumatic fractures in young HIV-infected patients would suggest that their fracture risk may indeed be higher,33-35 but further controlled, prospective studies are required to address this important question.
| Who should be screened for osteoporosis? | Top of page |
Routine screening of all HIV-infected patients with DXA is not recommended,36 especially in younger patients in whom the absolute risk of fragility fracture is low. Age, gender, and menopausal status are the most important factors to consider when making decisions regarding screening. All postmenopausal women older than 65 years should be offered screening, consistent with the current guidelines of the National Osteoporosis Foundation (
One of the most powerful predictors of future fragility fractures in the general population is a prior history of fragility fractures.37 Patients who have a history of atraumatic fracture (ie, a fracture resulting from a trauma equivalent to or less than that of falling from the standing position) or height loss (>2 cm) should be evaluated by DXA regardless of age, gender, or menopausal status. Currently, DXA scanning is vastly underutilized in this high-risk population. A retrospective study showed that only 10% of HIV-infected patients with documented fragility fractures received subsequent evaluation of bone density.38
Patients with osteopenia or osteoporosis should be evaluated for secondary causes of reduced BMD. The most cost-effective evaluation for secondary causes has not been established. Hyperparathyroidism, subclinical hyperthyroidism, hypogonadism, hypophosphatemia, idiopathic hypercalciuria, and vitamin D deficiency are conditions that are relatively common, result in reduced BMD, and are reversible with specific treatment. Vitamin D deficiency is particularly common. In a recent cross-sectional study of HIV-infected patients in Italy, 46% of those with reduced BMD were found to be vitamin D deficient, which was more than twice that of patients with normal BMD.39 A possible work-up for secondary causes is seen in Table 1. In selected cases, celiac disease, multiple myeloma, and Cushing’s syndrome should also be considered.
| Who should be treated? | Top of page |
All patients with osteopenia or osteoporosis should receive 1200 to 1500 mg of calcium daily and 400 to 800 IU of vitamin D, which should be ideally derived from the diet. In a dietary analysis of 125 HIV-infected patients, 70% did not reach their calcium intake goal as set by the US Recommended Daily Allowance.28 Weight-bearing exercise should also be recommended. Studies from postmenopausal women in the general populations show that 30 minutes of weight-bearing exercise at least 3 days per week improves BMD and reduces fracture.40 Lifestyle changes, such as the cessation of heavy alcohol use and smoking, should also be recommended.
| Table 1. Secondary Causes of Reduced BMD and Screening Modalities | |
| Condition | Screening Test |
| Basic Evaluation | |
Vitamin D deficiency | 25 OH vitamin D |
Hyperparathyroidism | PTH, Ca++ |
Hypogonadism | Testosterone (males); menstrual history (premenopausal females) |
Subclinical hyperthyroidism | TSH |
Idiopathic hypercalciuria | 24 h urinary calcium |
Phosphate wasting | Serum phosphate |
| Additional Testing | |
Celiac sprue | Tissue transglutaminase |
Multiple myeloma | Serum/ urine protein electrophoresis |
Cushing’s syndrome | Dexamethasone suppression test or equivalent |
The goal of pharmacologic treatment of osteoporosis is to reduce the risk of fractures and maintain function. All patients with a history of atraumatic fracture should be offered pharmacologic therapy after secondary causes have been evaluated and treated. In postmenopausal women, irrespective of HIV-infection status, the National Osteoporosis Foundation has established the following guidelines for the initiation of drug therapy (http://www.nof.org/physguide): 1) BMD T-scores below -2.0 by hip DXA with no risk factors or 2) BMD T-scores below -1.5 by hip DXA with 1 or more risk factors. In men and premenopausal women, it is reasonable to consider treatment for those with a T-score at or below -2.5. For those with less severe reductions in BMD (ie, osteopenia), therapy recommendations should be based on the presence of other risk factors and the degree of bone loss. If treatment is not given, serial DXA measurements (every 1-2 years) can be used to evaluate for the rapidity of change. Those patients who have a yearly decline of more than 5% should be considered for therapy.
Bisphosphonates prevent bone resorption by osteoclasts and are considered first-line therapy for both men and women. The newest-generation oral bisphosphonates (ie, risedronate, alendronate, ibandronate) have been shown to reduce the risk of fracture in patients without HIV infection. In a small randomized trial in HIV-infected patients, alendronate (70 mg/wk) increased lumbar spine BMD by 5.2% over 48 weeks accompanied by decreased bone turnover.41 Alternatively, annual intravenous (IV) zoledronate can also be used to maintain BMD in those who cannot tolerate oral therapy.42
Although well tolerated in most patients, the most frequent adverse effects of oral bisphosphonate therapy are esophageal irritation and dyspepsia. As a result, the drug should be administered with a full glass of water and the patient should be instructed to remain upright for at least 30 minutes. In addition, there are some concerns about long-term therapy with bisphosphonates, which can reside in the bone matrix many months after discontinuation. These include the oversuppression of bone turnover, increased mineralization (which may render bone more brittle), and the emerging risk of osteonecrosis of the jaw.43 Given the prolonged effects of bisphosphonates and the uncertainty surrounding their long-term safety, the need for lifelong therapy has been questioned. Some recommend discontinuation after 5 years, since additional benefits beyond this period have not been established.44
In postmenopausal women, osteoporosis can be effectively treated with estrogen-replacement therapy. However, because of the risk of breast cancer, endometrial cancer, cardiovascular disease, deep vein thrombosis, and Alzheimers’ disease, estrogen replacement therapy cannot be recommended as first-line treatment. The selective estrogen receptor modulator, raloxifene, is a reasonable alternative or adjunctive treatment to bisphosphonates in postmenopausal women. This drug has been shown to reduce the risk of fractures without increasing the risk of breast or endometrial cancer.37 No data are currently available on its use in HIV-infected patients.
Teriparatide, an analogue of parathyroid hormone (PTH), stimulates new bone formation by increasing the number and/or activity of the bone-forming osteoblasts and has been shown to reduce the risk of fracture. Its use should be restricted to those patients who continue to have fractures with bisphosphonate exposure, or those who are at a higher risk of fracture and not responding to bisphosphonates. The optimal treatment duration is unknown, but 12 to 24 months is generally recommended. It has not been specifically evaluated in HIV-infected patients.
Summary: Reduced BMD is common among HIV-infected patients and its etiology is multifactorial. Although prevention of fractures through targeted screening and early treatment is a reasonable strategy, the cost:benefit ratio has not been determined. Screening should be reserved for those patients with other risk factors of reduced BMD. In asymptomatic patients, treatment should be offered only to those with T-scores of less than -2.5 or other risk factors.
| Adrenal Insufficiency: | Top of page |
TF is 46-year-old white male with HIV since 1995. Current CD4 is 608 cells/ mm3, VL < 50 copies; he is receiving d4T/3TC/IDV. His HIV course has been complicated by lipoatrophy, and blindness secondary to cytomegalovirus (CMV) retinitis. He presented to a new HIV treatment provider complaining of weakness and dizziness when standing. On physical examination, he was afebrile, his blood pressure was 140/80 mm per Hg, his heart rate was 88 beats per minute without orthostatic changes. He was sunburned, but had a distinct tan line. Laboratory evaluation showed normal hemoglobin and serum electrolytes, including sodium and potassium. In addition, he was found to have an 8 AM cortisol of 0.5 µg per dL (normal: 6-26 µg/dL), which was confirmed on repeat measurement. Simultaneous adrenocorticotropic hormone (ACTH) was 2.2 pg per mL (normal: 5-27 pg/mL). Subsequent work-up included a normal pituitary MRI, normal iron studies, normal free thyroxine concentration and insulin-like growth factor-1. He was also found to have a serum testosterone of 171 ng per dL with a low FSH/LH and admitted to anabolic steroid use because of his fatigue. Upon further questioning, it was discovered that he had been receiving intermittent intraocular steroid injections to decrease the discomfort associated with CMV retinitis. He was started on hydrocortisone 20 mg in AM and 10 mg PM along with testosterone replacement with resolution of his symptoms.
| How is adrenal insufficiency classified? | Top of page |
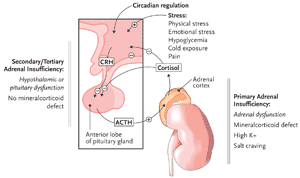
Figure 1. The Hypothalamic-Pituitary-Adrenal Axis
Adapted with permission from David Klemm. Am Fam Phys 2000;5:1119-1127.
In healthy individuals, corticotropin-releasing hormone (CRH) is secreted in pulsatile fashion by the hypothalamus in response to physiologic and psychologic stressors, as well as circadian stimuli, which then leads to ACTH secretion from the anterior pituitary. In response to ACTH, cortisol is released by the adrenal cortex, leading to alterations in cellular metabolism throughout the body. Cortisol also provides negative feedback to the hypothalamus and pituitary to limit secretion of CRH and ACTH (Figure 1).
Adrenal insufficiency (AI) is generally classified as primary (failure at the level of the adrenal gland), secondary (failure at the level of the pituitary), or tertiary (failure at the level of the hypothalamus). In practice, it is difficult and not clinically useful to attempt to differentiate hypothalamic from pituitary dysfunction, and these 2 problems are often grouped together as central AI (also somewhat confusingly called secondary AI).
| How is adrenal insufficiency diagnosed? | Top of page |
After the suspicion of AI is raised (Table 2), the first step in the evaluation is to confirm its presence. The optimal laboratory evaluation for AI, which balances sensitivity, specificity, cost effectiveness, and patient safety has not been clearly established. In the outpatient setting, a serum cortisol drawn between 8:00 and 10:00 AM (period of peak cortisol secretion in an individual with a normal sleep/wake cycle) can be a useful screening test.45 Serum cortisol of less than 3 gµ per dL is generally diagnostic of AI, whereas a value greater than 18 gµ per dL, rules out the diagnosis,46 although some recommend an upper cutpoint as low as 10 µg per dL.47 In my practice, I generally use a cutpoint of above 15 gµ per dL to rule out the diagnosis.
| Table 2. Clinical Clues Prompting Adrenal Insufficiency Evaluation | |
| Clinical Symptoms | Orthostatic symptoms (dizziness or lightheadedness on standing) Salt craving Unexplained fatigue or weakness Unexplained nausea, vomiting, or diarrhea |
| Clinical Signs | Hyperpigmentation Unexplained hypotension |
| Laboratory abnormalities | Hyperkalemia Hyponatremia Hypoglycemia |
| Associated Comorbidities | Anterior pituitary dysfunction |
| Other (specifically for exogenous steroid-induced ai) |
Cushingoid features Osteoporosis |
There are several caveats that need to be kept in mind in the measurement of serum cortisol. Since cortisol is mostly protein bound, changes in plasma proteins can affect the concentrations of the serum cortisol concentration, which is a reflection of both the free and protein-bound fractions. Estrogen, for example, increases cortisol-binding protein such that the total serum cortisol may not be an accurate surrogate of the bioactive free fraction. As a result, false-negative diagnoses may be more prevalent. Conversely, the serum cortisol may underestimate the free fraction in patients who have low plasma proteins, potentially leading to a false-positive diagnosis. Finally, a serum cortisol suggesting AI should be repeated before a diagnosis is established.
If the AM serum cortisol is intermediate (3-15 gµ/dL), an ACTH stimulation test should be performed. In the classic ACTH stimulation test, 250 mg of synthetic ACTH is given and cortisol is measured at 30 and 60 minutes. A peak stimulated cortisol of greater than 18 µg per dL rules out the diagnosis. Because the ACTH stimulation with the classic dose is supraphysiologic (approximately 1000 times normal), the use of a lower dose stimulus (1 mg) has been advocated to improve sensitivity, particularly in mild cases of central AI.48 There is controversy, however, regarding which dose should be used for routine evaluation. While some recommend the low dose test for all cases,45 others suggest that the high dose test should be used as first line.49 In HIV-infected patients, the optimal test is not clear. One study in HIV-infected patients showed poor agreement with the high and low dose tests.50 In my practice, the 250-mg test is generally used but, occasionally the 1-mg test is used for cases of suspected central AI with convincing symptoms.
In the setting of discordant or equivocal results, further testing is required. In the insulin tolerance test, a single dose of IV insulin (0.1 IU/kg) is given to bring the serum glucose to less than 40 mg per dL. A normal peak cortisol response is considered to be above 18 gµ per dL. This test should be performed at an experienced center and is contraindicated in older patients, those who have coronary artery disease, cerebrovascular disease, or seizure disorder. Metyrapone, an inhibitor of the final step of cortisol synthesis, can also be used to test the integrity of the cortisol axis. After a single midnight dose (30 mg/kg), a healthy patient will show a low cortisol level (<5 µg/dL) with a compensatory increase in ACTH and the cortisol precursor,11-deoxycortisol (>7 µg/dL) at 8:00 AM. Although no longer readily available in the United States, metyrapone can be obtained from the manufacturer for purposes of testing (Novartis Pharmaceutical Corp, 1-800-988-7768).
Once the diagnosis of AI is established, the next step is to differentiate a primary from a secondary disruption in the hypothalamic-pituitary-adrenal (HPA) axis. Measurement of ACTH is the single best test for this purpose. In primary AI, the ACTH level is very high (>100 pg/mL; normal 20-52 pg/mL) due to the absence of negative feedback on the hypothalamus and pituitary. In secondary AI, the ACTH concentration is low or in the low-normal range. For practical purposes, it is reasonable to obtain the ACTH level at the same time as repeat basal cortisol testing or prior to an ACTH stimulation test to facilitate the work-up.
Other clues can help to differentiate primary from secondary AI. In primary AI, but not in secondary AI, mineralocorticoid function is affected. As a result, salt craving and hyperkalemia may be present. In addition, hyperpigmentation may be present in primary AI since ACTH and melanocyte-stimulating hormone are derived from a common precursor. Primary AI due to autoimmune adrenalitis may be associated with other autoimmune diseases (eg, hypothyroidism, vitiligo, type 1 diabetes mellitus). Secondary AI may be associated with other anterior pituitary deficits.
| What are the causes of adrenal insufficiency in HIV-infected patients? | Top of page |
After the diagnosis of AI has been made and the level of defect has been determined, the final step is to determine the underlying cause. Table 3 shows the most common causes of AI in HIV-infected patients.
In patients with advanced AIDS, primary AI can be caused by adrenal gland infection.51 Infective agents associated with AI include tuberculosis,52 CMV,53,54 Cryptococcus,55 Nocardia,56 HIV itself,57 Mycobacterium avium-intracellulare,55 and Histoplasma capsulatum.58 Infiltration of the adrenal gland by malignant tumor either unrelated or related to immunosuppression (Kaposi’s sarcoma or lymphoma) can also lead to primary AI. Medications causing primary adrenal dysfunction which may be more frequently used in HIV-infected patients include ketoconazole, fluconazole59 and rifampin.60 Similar to the general population, primary AI may also result from adrenal hemorrhage or autoimmune adrenalitis.
| Table 3. Causes of AI in HIV-infected Patients | |
| Primary AI | Secondary/Tertiary AI |
Infection
Autoimmune Hemorrhage Medications
|
Infection/Infiltration
Isolated ACTH Deficiency Tumor Trauma Medications
|
Secondary AI may be caused by hypothalamic or pituitary disease including infiltration, infection, tumor, or trauma. Megesterol, a progestational agent with glucocorticoid activity frequently used for HIV-associated wasting, has been associated with AI61 which will likely reverse with discontinuation. Exogenous glucocorticoids are perhaps the single most common reason for suppression of the HPA axis for adrenal suppression in the HAART era and deserve additional discussion.
Glucocorticoid-induced adrenal suppression in HIV disease: In the HAART era, there have been increasing reports of AI among HIV-infected patients in the setting of glucocorticoid exposure. Most of these reports have been in patients receiving inhaled fluticasone with concomitant HAART,62-68 although AI with inhaled budesonide has also been reported.69 In addition, adrenal suppression with topical steroids has been described.70 I have treated 3 other patients with AI likely due to intermittent, local steroid injections (intra-ocular [above case], intra-articular [hip], and soft tissue [for plantar fasciitis]).
The cause of the increase in adrenal suppression appears to be a pharmacologic interaction between glucocorticoids and antiretrovirals. The principal metabolic route of glucocorticoids is via the cytochrome P450 enzyme CYP3A4, which is also inhibited by some HIV protease inhibitors, including low-dose ritonavir. Ritonavir, therefore, also can “boost” the concentrations of steroids leading to systemic effects using doses and routes of administration previously thought to have minimal adverse effects.
Many of the details regarding the glucocorticoid-ritonavir interaction have not been clarified. Among the inhaled steroids, differences in their capacity to suppress the HPA axis have not been investigated. Cases involving fluticasone and budesonide have been described, but not with other inhaled steroids (flunisolide, beclomethasone, triamcinolone, or mometasone). However, the safety of these other compounds in the setting of ritonavir has not been specifically evaluated. Mometasone, for example, is extensively metabolized by CYP3A4 and, therefore, a pharmacologic interaction may be predicted.71 Among oral steroids, hydrocortisone theoretically has the most potential for interaction with CYP3A4 compared with dexamethasone and prednisone, but a recent pharmacokinetic study showed that prednisolone (an active metabolite of prednisone) concentrations are increased by 30% with low-dose ritonavir coadministration.72 To my knowledge, data regarding the magnitude of interactions with topical or local steroids have not been described.
Adrenal suppression from exogenous glucocorticoids has 2 general patterns of presentation. First, if the steroids are being continuously given, iatrogenic Cushing’s syndrome may develop, with central adiposity, facial plethora, dorsocervical fat pad, glucose abnormalities, skin fragility, osteoporosis, and/or emotional lability. At the end of the dosing interval or if the corticosteroids are withdrawn, symptoms of AI can appear, including fatigue, dizziness when standing, and lassitude. The body composition changes share some of the features with HIV-related lipodystrophy and, as a result, the proper diagnosis may be delayed.73,74 Secondly, some patients may not develop classic signs and symptoms of Cushing’s syndrome, but may only have symptoms of AI. This is the most common scenario when steroids are given intermittently. Endogenous adrenal function generally returns and symptoms of Cushing’s regress with discontinuation of the steroids or ritonavir, but some patients may need to be supported with oral glucocorticoids (see below).
| How should adrenal insufficiency be treated? | Top of page |
Glucocorticoid replacement is essential for all patients with symptomatic AI. Typically, hydrocortisone (20-30 mg/d in 2 divided doses), prednisone (5 mg/d), or dexamethasone (0.5-0.75 mg/d) is prescribed. Given the potential for pharmacologic interaction, it is not known whether doses should be reduced if the patient is also receiving ritonavir as part of a HAART regimen. To avoid adrenal crisis, patients should be instructed about the significance of their diagnosis, should wear MedicAlert identification, and should triple their steroid dose if they become ill. Stress doses of steroids (eg, hydrocortisone 50-100 mg 3 times daily) should be administered during major illness or surgery. For those patients with primary AI who also lack mineralocorticoids, fludrocortisone should be administered at a dose high enough to eliminate orthostatic symptoms and normalize serum potassium and plasma renin activity.
Treatment considerations in glucocorticoid-induced adrenal suppression: The first step is to determine whether steroids are required for the condition for which they were originally prescribed. If so, switching steroids may be helpful, but alternatives with fewer interactions with antiretrovirals have not been clearly determined. For inhaled steroids, beclomethasone and flunisolide theoretically have fewer interactions. The safest topical or locally used steroids have not been determined. Switching HAART regimens may also represent a viable option for some patients.
If the steroids can be withdrawn and the patient is having symptoms, I generally give oral steroids at physiologic doses and taper very slowly (prednisone 5 mg qd, dexamethasone 0.5 mg qd). Periodically, I check an AM cortisol 24 hours after the previous steroid dose to gauge whether adrenal function is returning. A value greater than 5 µg per mL may prompt an ACTH stimulation test. If the patient is not experiencing significant symptoms, it is reasonable not to treat with oral steroids and to carefully monitor symptoms and adrenal function. Such patients should have oral steroids on hand, only to be administered at 3 times the physiologic dose during illness.
Summary: Adrenal insufficiency should be considered in HIV-infected patients with suggestive symptoms, particularly those with glucocorticoid exposure. A step-wise diagnostic strategy should be followed prior to initiation of therapy: 1) confirm the diagnosis, 2) distinguish primary from central AI, and 3) investigate the underlying cause. In many cases, consultation with an experienced endocrinologist can be helpful.
| Conclusions | Top of page |
The care of HIV-infected patients has become increasingly complex. Endocrine problems, such as osteoporosis and AI, have been frequently reported in the HAART era. Additional considerations may be required regarding the etiologies, diagnosis, and treatment compared with the general population. Further research is required to understand the intricacies of these problems in HIV-infected patients in order to provide optimal care.
| References | Top of page |
