Nearly all primary care providers in the United States, especially those with sizeable HIV practices, are aware of the very real dangers of crystal methamphetamine use. It has been a pervasive concern among many Physicians’ Research Network members—indeed, a hot topic during several question-and-answer sessions—and an issue that PRN has been struggling to adequately address.
PRN’s first agenda item was to find a speaker to lay out some of the potential physiologic concerns of crystal meth use among HIV-positive people. We were promptly guided to Dr. Scott Letendre of the University of California, San Diego, who has been engaged in some significant research involving the effects of methamphetamine on central nervous system function in HIV-positive people. We present here a summary of his June 2005 lecture and intend to publish additional articles in coming Notebook issues, to increase awareness among clinicians regarding the very real dangers of methamphetamine addiction and its potential management.
| I. Crystal Methamphetamine: An Introduction | Top of page |
The ease with which crystal meth can be manufactured is a major contributing factor to the increase in its use. Law enforcement officials identify and close hundreds of clandestine methamphetamine labs each year. Large operations produce methamphetamine in Mexico and California. Outside of these areas, small rural laboratories are more common. Rural areas are favored because strong odors are produced in the process of “cooking” the drug, which can draw immediate attention to the manufacturing operation.
The manufacturing of crystal meth begins with large quantities of ephedrine or pseudoephedrine, which are found in numerous over-the-counter cold medications (manufacturers of these compounds are currently looking into producing pseudoephedrine-free products). These are then cooked with a variety of other readily available substances, including red phosphorus, hydrochloric acid, drain cleaner, battery acid, lye, paint thinner, Freon, and/or antifreeze (every pound of crystal meth produced leaves behind five to six pounds of toxic waste). And because it is so easily and rapidly produced, it remains one of the cheapest drugs available.
With the manufacturing process completed, crystal meth is bagged and sold as shiny yellowish-white rocks in various sizes or as a crystalline powder resembling small fragments of glass. It can be ingested by snorting or smoking, dissolved in water to be swallowed or inserted into the rectum (“booty bump”), or injected intramuscularly or intravenously. On the street, it goes by a variety of names, including crystal, glass, crank, Tina, Crissy, and ice (in its rock form).
Figure 1.Methamphetamine Administration: Geographic Variations
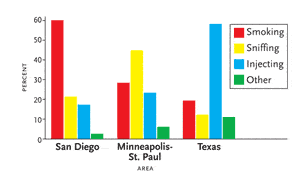
The preferred method of taking methamphetamine varies among geographic regions. The bar graph is based on surveys conducted by the National Institute of Drug Abuse Community Epidemiology Work Group in three U.S. regions: San Diego (survey conducted between July and December 2000), Minneapolis/St. Paul (survey conducted throughout 2000), and Texas (survey conducted in January through June 2001).
Source: NIDA Community Epidemiology Work Group
“The preferred route of administration varies by geographical region,” Dr. Letendre commented (see Figure 1). “In San Diego, people have learned that smoking meth gets the drug into the system quickly. In Texas, the preferred route is intravenous administration. In New York, the preferred route seems to be intravenous injection as well.” According to the National Institute of Drug Abuse (NIDA), the bioavailability of methamphetamine administered via the oral route is 67.2%. Through the intranasal route, the bioavailability is 79%. Smoking it increases the bioavailability to 90.3% and injecting it intravenously increases the bioavailability to 100%
Plasma concentrations of methamphetamine peak approximately 15 minutes after it is administered. Concentrations drop by approximately 50% within 30 minutes, followed by steady-state concentrations for another 90 minutes or so. “However, if we follow the concentration curve, we see that the drug remains in the system for more than 24 hours,” Dr. Letendre explained. “Metabolites in the urine can be detected out to three or four days.”
Methamphetamine is hepatically metabolized via deamination, conjugation, and hydroxylation. It is metabolized to amphetamine through cytochrome P450 2D6. “One potential concern here is the effect of genetic variation in 2D6 on methamphetamine concentrations,” Dr. Letendre commented. “Such genetic variations can result in poor and extensive metabolizer phenotypes.” Also of concern is the fact that ritonavir (Norvir) inhibits 2D6 and can result in threefold to tenfold increases in methamphetamine concentrations. “Several deaths have been reported from possible methamphetamine-protease inhibitor interactions,” he warned.
| Methamphetamine and the Brain | Top of page |
Methamphetamine penetrates the brain and cerebrospinal fluid (CSF) rapidly because of its lipid solubility. Once sequestered in the central nervous system, amphetamines act as “false” neurotransmitters. The drug stimulates release of dopamine, serotonin, and norepinephrine. The flood of these neurotransmitters amplifies sensory perceptions and induces a long-lasting rush of euphoria, energy, elation, and confidence. However, what goes up must come down. Acute and chronic use of amphetamine leads to injury of dopaminergic, glutaminergic, and serotonergic neurons.
Dopamine is a catecholamine that can modulate posture and movement, emotion and motivation, and attention and other cognitive processes. “Dopamine pathways are injured in Parkinson’s disease, attention-deficit/hyperactivity disorder (ADHD), and schizophrenia,” Dr. Letendre said.
Glutamate is an amino acid that is primarily excitatory. It has multiple receptors, including NMDA receptors that are broadly distributed in the hippocampus and neocortex. Up to 90% of glutamate synapses are found at dendritic spines, usually on postsynaptic neurons. Glutamate overstimulation (excitotoxicity) can cause neuronal death. “Patterns of glutamate transmission can lead to neuronal remodeling, which may undermine learning and memory,” Dr. Letendre explained. “Glutamate-mediated injury occurs in stroke, epilepsy, motor-neuron diseases like ALS, Alzheimer’s disease, Huntington’s disease, and HIV-associated dementia.”
Serotonergic neurons are confined to the brainstem but send axons throughout the brain and spinal cord. They are responsible for the euphoria associated with methamphetamine, which has among the highest addiction potentials when snorted or smoked. Damage can have extensive effects, including aggression, hypersexuality, and learning deficits. “Injury has been implicated in depression, alcoholism, Alzheimer’s disease, schizophrenia, autism, anorexia nervosa, OCD, and Downs syndrome,” Dr. Letendre said.
There is also the possibility of vascular injury. In animals, methamphetamine can cause cerebral ischemia and infarction, vasculitis of the small arteries, and cerebral edema. And in humans, many cases of hemorrhagic and ischemic stroke have been reported in association with methamphetamine use, as well as cardiovascular collapse. Angiitis in multiple organs—including the brain—has been reported, as have cases of rhabdomyolysis.
| Methamphetamine and HIV | Top of page |
When it comes to methamphetamine and HIV, Dr. Letendre contends that there are potentially multiple levels of interaction (see Figure 2). First and foremost is the increased risk of acquiring HIV and other sexually-transmitted infections. Various research teams have documented that, when crystal meth is used in association with sexual activity, condoms are more likely to be abandoned, numerous sex partners are more likely to be had, and trauma to the lining of the anus and/or vagina is more likely to be experienced.
Figure 2.Methamphetamine and HIV: Multiple Interactions
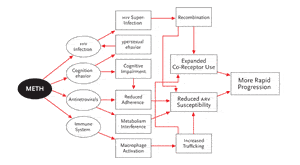
A schematic representation of the potential effects methamphetamine use can have on HIV disease. For example, methamphetamine use can increase the risk of acquiring HIV infection or, in people already infected with the virus, superinfection. Superinfection can result in recombination, leading to expanded coreceptor use, and ultimately to more rapid disease progression. Also suggested here is an effect of methamphetamine on macrophage expression, resulting in increased expression of adhesion molecules. This potentially leads to increased trafficking across anatomic compartments, between the blood and the central nervous system and genital tract. With increased trafficking, HIV is exposed to a wider variety of cells and cell receptors, as well as lower concentrations of antiretrovirals (particularly in the CNS and genital tract), resulting in reduced antiretroviral susceptibility and more rapid disease progression.
Source: Scott Letendre, MD
Recent data collected by the Center for HIV/AIDS Education Studies and Training (CHEST) at New York University indicate that, in New York City, men who have sex with men (MSM) who use methamphetamine are three times more likely to contract HIV through receptive anal intercourse than MSM who do not use the drug (Halkitis, 2005). Moreover, the CHEST study found that among gay or bisexual male “party drug” users, approximately 62% indicated significant and frequent use of crystal methamphetamine. This is an increase from the early 1990s when usage rates among gay and bisexual men ranged between 5% and 25%. CHEST also found that MSM who reported crystal meth use were diverse in terms of ethnicity, age, income, and HIV status; 45% of the samples were men of color, and half the men reported being HIV-positive.
Researchers with the San Francisco Department of Public Health (SFDPH) have reported that MSM who used crystal and Viagra together were six times more likely to be diagnosed with syphilis than those who didn’t use either drug (Wong, 2004). In another study from SFDPH, researchers found that 17.4% of 1,263 MSM who attended the city’s public STD clinic had used crystal in the month before their visit. Those who used the drug were more than twice as likely as non-users to be HIV positive, four times as likely to be diagnosed with syphilis, and almost twice as likely to test positive for gonorrhea (Mitchell, 2004).
Some experts argue that these numbers may only be the tip of the iceberg. Presently, most city and state departments of health do not routinely track crystal meth use among people newly infected with HIV, leading to an incomplete picture of the extent of the role of crystal meth use on HIV infection statistics.
Among methamphetamine users infected with HIV, there are additional issues to consider. First there is an inflammatory component. Methamphetamine can upregulate expression of chemokines and cytokines, including tumor necrosis factor-alpha (TNF-a). Since pro-inflammatory cytokines can upregulate HIV replication, this can contribute greatly to a second component: worse control of HIV disease. Methamphetamine may also have a negative effect on antiretroviral treatment. Reduced adherence is a common situation in methamphetamine users. There is also the possibility of negative drug interactions, resulting in substandard antiretroviral concentrations in the body, which can lead to antiretroviral resistance.
Last but not least is the dual effect of HIV and methamphetamine on the brain. For example, methamphetamine can increase feline immunodeficiency virus (FIV) replication and mutation in feline astrocytes. According to one study, FIV replication increased by as much as 15-fold (Gavrilin, 2002). Striatal dopamine content has also been evaluated in rats exposed to HIV tat, methamphetamine, or both (Maragos, 2002). In rats exposed to tat alone, there was a 16% decrease in dopamine content. In rats exposed to methamphetamine alone, there was a 40% decrease in dopamine content. And in rats exposed to HIV tat and methamphetamine together, there was a whopping 75% decrease in dopamine content. “These data don’t take into consideration that, in addition to HIV tat, HIV env—gp120—can also injure dopaminergic neurons,” Dr. Letendre added.
| II. UCSD Findings | Top of page |
To shed some more light on the interaction between methamphetamine and HIV in the CNS, the HIV Neurobehavioral Research Center at UCSD has been investigating whether—and by what mechanisms—methamphetamine use enhances brain injury in the context of HIV infection. The overall design of the UCSD project is a two-by-two factorial, longitudinal, observational study. Subjects are enrolled into four categories, defined by the presence or absence of HIV infection and methamphetamine dependence. For the sake of this study, methamphetamine use is defined as “dependence” within 18 months, based on DSM-IV criteria. Subjects with concomitant cocaine and/or heroin use are excluded and only a limited use of alcohol is allowed (no dependence or abuse allowed). “This is not an interventional study,” Dr. Letendre commented. “The project did not aim to provide treatment. Instead, we set out to observe differences in the patient populations that were linked to HIV infection and meth use.”
Dr. Letendre first reviewed data involving 398 study subjects. Ninety-two subjects were HIV-positive with a history of methamphetamine use (HIV+/meth+), 105 were HIV-positive without a history of methamphetamine use (HIV+/meth-), 115 were HIV-negative with a history of methamphetamine use (HIV-/meth+), and 86 were HIV-negative without a history of methamphetamine use (HIV-/meth-). “We tried to match the ages and the education levels,” Dr. Letendre explained. “However, the HIV-positive subjects and the methamphetamine-using subjects tended to be older than the HIV-negative subjects not using methamphetamine. We also found that the methamphetamine users were somewhat less educated than the subjects not using methamphetamine. The methamphetamine users completed 11 or 12 years of education, whereas those not using methamphetamine were more likely to have completed some college. We also worked hard to enroll women, but we were only variably successful; in San Diego, methamphetamine use is much more prevalent in men than in women. The same also held true with racial demographics, with methamphetamine use being more prevalent among white study subjects.”
Dr. Letendre went on to discuss neuropsychological differences between the four groups. Neuropsychological test results were summarized using the global deficit score (GDS), a validated measure that accounts for performance in seven cognitive domains and adjusts for age, education, and ethnicity. “This measure does not give points for supranormal performance,” Dr. Letendre explained. “It ranges between zero and five and is roughly interpretable as the number of standard deviations below which someone performed relative to their matched controls. We think a level of .5 or higher reliably signifies impairment.”
At baseline, the GDS was 0.28 in the HIV-/meth- group, which is less than the 0.5 cutoff for neuropsychological impairment. In the HIV+/meth- and HIV-/meth+ groups, the GDS was 0.54 and 0.52 respectively, indicating some degree of neuropsychological impairment. And in the HIV+/meth+ group, the GDS was 0.65, indicating a greater degree of impairment among the HIV-positive methamphetamine users. When analyzed as a dichotomous variable (i.e., impaired or normal), approximately 53% of subjects in the HIV+/meth+ group had neuropsychological impairment, compared to 37% each in the HIV+/meth- and HIV-/meth+ groups, and 20% in the HIV-/meth- groups. “These differences were highly statistically significant,” Dr. Letendre said. “More specifically, we’ve documented that the HIV-positive and HIV-negative methamphetamine users are more likely to experience impaired abstraction, learning, recall or memory, motor function, and attention than those in the other groups. The GDS is dependent on several domains and we see significant differences in many of these domains when comparing the methamphetamine users to the others.”
As this is a longitudinal study, follow-up neuropsychological testing data are also available. According to Dr. Letendre, approximately 18% of the subjects in the HIV+/meth+ group had evidence of neuropsychological worsening during at least one follow-up, compared to 12% in the HIV-/meth+ group, 11% in the HIV+/meth- group, and 7% in the HIV-/meth- group. “Not only do HIV and methamphetamine confer independent risk of brain injury in a cross-sectional analysis,” Dr. Letendre said, “they also confer risk of progression in a longitudinal analysis.”
| Methamphetamine and Biological Markers | Top of page |
Dr. Letendre’s group has also evaluated plasma and CSF HIV-RNA levels in HIV-positive individuals with a history of methamphetamine use (Ellis, 2003). Three groups were evaluated: 88 subjects with a history of methamphetamine use with evidence of recent use, determined by positive toxicology results (meth+/tox+); 66 subjects with a history of methamphetamine use with no evidence of recent use (meth+/tox-); and 76 subjects without a history of methamphetamine use and no evidence of recent use (meth-/tox-). More than half of the subjects were receiving antiretroviral therapy at the time of study entry.
Figure 3.Relationship of Antiretroviral Therapy to Plasma HIV-RNA Levels in Three Study Groups
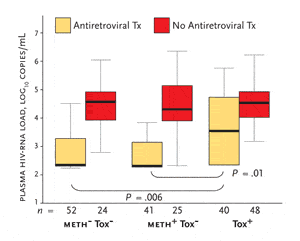
Box-and-whisker plots show the median (center line), interquartile range (box) and 5th and 95th percentiles (whiskers) for subjects receiving antiretroviral therapy and for those not receiving antiretroviral therapy. Meth-/tox-: no history of methamphetamine dependence or current use; meth+/tox-: history of methamphetamine use but no current use; meth+/tox+: history of methamphetamine use and evidence of recent use.
Source: Ellis, 2003. The Journal of Infectious Diseases 188:1822. Adapted with permission of the Infectious Diseases Society of America.
The meth+/tox+ patients had plasma HIV-RNA levels that were significantly higher than either the meth+/tox- or meth-/tox- patients. However, adjustment for antiretroviral use identified that the only significant differences were among the subjects receiving treatment (see Figure 3). Compared to subjects receiving antiretroviral therapy in the meth+/tox- and meth-/tox- groups, subjects receiving antiretroviral therapy in the meth+/tox+ had significantly higher viral loads. In contrast, HIV-RNA levels among meth+/tox+ subjects not receiving antiretroviral therapy were not significantly higher than those in the meth+/tox- or meth-/tox- groups. This, Dr. Letendre suggested, argues against a direct biological effect of methamphetamine use itself on viral replication. Rather, it suggests that recent methamphetamine use and antiretroviral therapy interact in their effects on viral load. This, he said, “is no less of a significant problem.”
No evidence of increased CSF viral load in meth+/tox+ patients was documented, compared to meth+/tox- or meth-/tox- study volunteers.
The increases in plasma viral load found among the meth+/tox+ subjects receiving antiretroviral therapy may be due to poor adherence or to altered metabolism of antiretroviral medications. This study, however, was unable to determine the exact cause. Dr. Letendre’s group collected self-reports of adherence and found them to be similar in the meth+/tox+ subjects, compared with subjects in the other groups. However, he noted, self-reports of adherence to therapy can be inaccurate.
Dr. Letendre also pointed out that, at baseline, the duration of methamphetamine abstinence correlated with HIV-RNA levels in plasma and in CSF. And in the longitudinal analysis, relapsing methamphetamine users had higher HIV-RNA levels in plasma (4.4 log10 copies/mL vs. 2.3 log10 copies/mL) and in CSF (2.4 log10 copies/mL vs. 1.7 log10 copies/mL) compared with those who remained abstinent.
Additional data from UCSD indicate that methamphetamine use is associated with increased inflammation in HIV-positive individuals. Dr. Letendre explained that HIV-positive methamphetamine users had higher levels of five markers of macrophage activation—including MCP-1, sCD14, sTNFR-II, TNF-a, and MIP-1 beta—in plasma. Three markers were also higher in the CSF of methamphetamine users. And similar to HIV-RNA levels, macrophage activation levels varied with recency of methamphetamine use.
| Methamphetamine and Neurobiology in HIV | Top of page |
Dr. Eliezer Masliah, Professor of Neuroscience and Pathology at UCSD, and his colleagues have been conducting a great deal of neurobiological research surrounding the effects of methamphetamine in the brains of HIV-positive individuals. His team’s research has focused on tissue samples collected at autopsy, which includes deceased individuals enrolled in the UCSD program project and samples retrieved from the National NeuroAIDS Tissue Consortium, which includes sites in San Diego, New York, Los Angeles, and Galveston.
To date, Dr. Masliah’s group has analyzed data from human brain tissue studies, animal studies, and in vitro experiments. Dr. Letendre reported on the human studies that used brain tissue from 19 HIV-negative volunteers (controls), some of whom had a history of methamphetamine use; 42 HIV-positive people with no history of methamphetamine use, and 27 HIV-positive people with a history of methamphetamine use (Langford, 2003). Samples were collected from patients who died around 40 years of age. A number of the HIV-positive patients had evidence of HIV encephalopathy at the time of death.
Overall, HIV-positive meth users more frequently had evidence of ischemic events, severe inflammation (as measured by microglial reactivity), and loss of synapses (as measured by synaptophysin) than HIV-positive people who did not use meth. While neuronal and dendritic structures were preserved in the control individuals, meth users who did not have HIV encephalitis (HIVE-negative) had moderate neuronal and dendritic damage, non-meth users who had HIVE had moderate-to-severe damage, and individuals who had both HIVE and meth use had the most severe damage. This last group also had extensive loss of calbindin-immunoreactive interneurons. This is important because loss of these interneurons interferes with neurotransmission of other neurons and has been implicated in other neurologic disorders, such as schizophrenia (Eyles, 2002) and motor neuron disease (Maekawa, 2004). “We have neuropsychological test scores for these patients, conducted before their death and the donation of brain tissue,” Dr. Letendre said. “Dr. Masliah’s group found that greater loss of interneurons among the HIV-positive methamphetamine users correlated with worse neuropsychological performance prior to death.”
These data—along with those being generated by ongoing studies—indicate that methamphetamine and HIV independently injure neural cells, such as microglia, and neurons by several mechanisms, leading to impaired cognition among HIV-positive methamphetamine users.
| III. Suggested Clinical Approaches | Top of page |
Dr. Eliezer Masliah, Professor of Neuroscience and Pathology at UCSD, and his colleagues have been conducting a great deal of neurobiological research surrounding the effects of methamphetamine in the brains of HIV-positive individuals. His team’s research has focused on tissue samples collected at autopsy, which includes deceased individuals enrolled in the UCSD program project and samples retrieved from the National NeuroAIDS Tissue Consortium, which includes sites in San Diego, New York, Los Angeles, and Galveston.
To date, Dr. Masliah’s group has analyzed data from human brain tissue studies, animal studies, and in vitro experiments. Dr. Letendre reported on the human studies that used brain tissue from 19 HIV-negative volunteers (controls), some of whom had a history of methamphetamine use; 42 HIV-positive people with no history of methamphetamine use, and 27 HIV-positive people with a history of methamphetamine use (Langford, 2003). Samples were collected from patients who died around 40 years of age. A number of the HIV-positive patients had evidence of HIV encephalopathy at the time of death.
Overall, HIV-positive meth users more frequently had evidence of ischemic events, severe inflammation (as measured by microglial reactivity), and loss of synapses (as measured by synaptophysin) than HIV-positive people who did not use meth. While neuronal and dendritic structures were preserved in the control individuals, meth users who did not have HIV encephalitis (HIVE-negative) had moderate neuronal and dendritic damage, non-meth users who had HIVE had moderate-to-severe damage, and individuals who had both HIVE and meth use had the most severe damage. This last group also had extensive loss of calbindin-immunoreactive interneurons. This is important because loss of these interneurons interferes with neurotransmission of other neurons and has been implicated in other neurologic disorders, such as schizophrenia (Eyles, 2002) and motor neuron disease (Maekawa, 2004). “We have neuropsychological test scores for these patients, conducted before their death and the donation of brain tissue,” Dr. Letendre said. “Dr. Masliah’s group found that greater loss of interneurons among the HIV-positive methamphetamine users correlated with worse neuropsychological performance prior to death.”
These data—along with those being generated by ongoing studies—indicate that methamphetamine and HIV independently injure neural cells, such as microglia, and neurons by several mechanisms, leading to impaired cognition among HIV-positive methamphetamine users.
“One thing I’m frequently asked,” Dr. Letendre said, “is what can we do in the clinic? One of the most important things we can do is to remember to ask our patients about their thinking and memory. In the course of a busy visit, it’s easy to get distracted from asking these basic ones. But, the fact is that we won’t identify cognitive symptoms if we don’t ask the right questions.” He recommends asking about work and activities associated with daily living. He also suggested corroborating with friends, partners, and parents.
Optimizing adherence and antiretroviral penetration into the CNS are very important, especially among individuals who have evidence of neuropsychological impairment. “We also want to quickly and effectively diagnose comorbidities, especially those that can result in neurological impairment,” he said, referring to the data linking HCV infection, heavy alcohol intake, depression, and anemia to poor neuropsychological functioning in HIV-positive people. “Also of major importance, he added, “is supporting abstinence to methamphetamine and other drugs, including alcohol.”
Dr. Letendre finished his lecture with a brief review of some therapies that may have neuroprotective properties and may be useful in overcoming the neuropsychological effects of methamphetamine addiction. “For the most part, these therapies are unproven,” he said, indicating that much more research is needed in terms of better understanding the utility of these compounds in the setting of HIV and methamphetamine use.
“We know that methamphetamine injures dopamine pathways,” Dr. Letendre reiterated. “In turn, drugs that modulate dopamine might be beneficial.” Unfortunately, some agonists—such as bromocriptine and pergolide—have not been effective in treating cocaine users. While cocaine injures the brain somewhat differently than methamphetamine, there are some similarities, including dopaminergic neuronal injury.
Drugs such as methylphenidate and bupropion—stimulants and indirect dopamine agonists—may play a role in methamphetamine addiction treatment. However, as is noted in a paper published by Dr. Antonio Urbina of St. Vincent’s Hospital and Medical Center and Dr. Kristina Jones of New York Presbyterian Hospital, methylphenidate has not been shown to decrease cocaine use but was associated with a lower dropout rate from drug abuse treatment than was placebo (Urbina, 2004).
There has also been a study of the NMDA antagonist memantine, in a study for HIV-associated neurocognitive impairment. “The results were not strongly positive, but memantine may provide benefit in a subgroup of patients.”
If serotonin pathways are injured, SSRIs may be beneficial. “In our CHARTER [CNS Antiretroviral Therapy Effects Research] study,” Dr. Letendre said, “we found that approximately 20% to 25% of our clinic patients are taking SSRIs. However, there are drug interactions to consider here.”
Also being explored are growth factors, such as erythropoietin (Procrit), to combat anemia-associated neurological deficits, along with anti-inflammatories, such as minocycline, NSAIDs, glutathione, and L-carnitine. “Inflammation can result from methamphetamine use and plays an important role in the progression of neurological decline in HIV,” Dr. Letendre said. “Therefore, using these agents to halt inflammation and, hopefully, neurological impairment, may be beneficial.”
| References | Top of page |
