For numerous HIV-infected patients, facial lipoatrophy has become a frustrating reality. While not typically life-threatening, it can lead to comorbidities and is one of the most stigmatizing complications of HIV. And because facial lipoatrophy is believed to be an adverse effect of antiretroviral therapy, it can significantly affect a patient’s “relationship” with his or her regimen, potentially resulting in poor adherence or termination of therapy altogether, even if the regimen is achieving a desired effect on viral load and CD4+ cell counts.
While it is true that mechanism(s) by which lipoatrophy occur have not been concluded, progress is at hand. For example, a number of cosmetic modalities are being explored—and used—for the correction of facial lipoatrophy. For Dr. Jeffrey Roth, who has consulted with numerous HIV-infected patients dealing with lipoatrophy, the selection of the right product has been something of a difficult task and requires knowledge of the advantages and drawbacks of each approach.
“One chief principle that cannot be overlooked when thinking about these products is primum non nocere,” Dr. Roth said, referring to the Latin phrase: first, do no harm. “We owe it to our patients not do them any harm. Lipoatrophy is a condition in which people tend to become very desperate to receive treatment. I think we need to be especially cautious that we don’t do any long- or short-term harm to our patients in attempting to meet their demands.”
| I. Lipoatrophy Primer | Top of page |
Lipoatrophy of the face is the primary focus of this article, although it can also occur in the arms, legs, and buttocks. Rarely, lipoatrophy can contribute to other complications, such as seborrheic dermatitis, decubitus ulcers, and palmar and plantar pain. There are psychological ramifications as well, including depression, social withdrawal, and immediate identification as a person with HIV/AIDS.
Facial fat is distributed throughout the face as subcutaneous fat, in fat pockets around the orbits, and in buccal and temporal fat pads. Facial lipoatrophy is characterized by fat loss through the face and occurs most commonly in the cheeks, temples, nasolabial regions, and—in severe cases—the orbits. Additional physical findings include diminished subcutaneous fat, visceral fat accumulation, “buffalo hump,” and parotid cystic hyperplasia.
While there are a number of potential mechanisms of HIV-associated lipoatrophy, there is little consensus regarding the exact cause(s). Contributing factors may include mitochondrial toxicity, lactic acidosis, insulin resistance, direct drug effects, and genetic factors.
Adipocytes in Lipoatrophy: Depleted or Dead?
Is lipoatrophy permanent? This, Dr. Roth explains, is an extremely important question that requires answering if we are to truly understand how best to manage it. “We used to think that T-cells only went down and didn’t go up,” he said. “Then we proved that they could increase, but still believed that they weren’t functional T-cells. The fact of the matter is, there are a number of aspects of HIV disease that we thought could only get worse as time went on, but have been proven wrong. The same thing might be true for lipoatrophy, although we don’t know enough about the precise mechanism to say whether it’s permanent or not.”
Forecasting whether or not lipoatrophy is permanent depends on the answer to a fundamental question: Are subcutaneous and fat-pad adipocytes depleted, or dead? “If there’s apoptosis of the adipocytes, or if the fat cells are dead, then the chances that fat will reaccumulate once the metabolic defect is corrected, are pretty small,” Dr. Roth commented. “But if the fat cells are viable and the metabolic defect is corrected, a patient’s normal fat distribution may return.”
In a paper reviewed by Dr. Roth, investigators in Barcelona perfomed an ultrastructural study of adipocytes from 10 HIV-infected patients who received antiretroviral therapy for 20 to 42 months (Lloreta, 2002). In short, the investigators suggested that antiretroviral therapy-associated lipodystrophy, including lipoatrophy, is the result of a complex remodeling process of adipocytes involving variable combinations of apoptosis, defective lipogenesis, and increased metabolic activity in different adipose areas of the body. In other words, there may be both reversible and irreversible components to lipoatrophy.
In another study, reported by investigators at the State University of New York at Stony Brook, the rate of apoptosis in subcutaneous adipose tissue from HIV-negative study volunteers and HIV-positive patients with and without lipoatrophy was examined using a sensitive ligase-mediated PCR technique to amplify DNA ladders (Mynarcik, 2005). Although apoptosis was observed in subjects with HIV-associated lipoatrophy, there was no difference in the incidence of individuals with apoptosis among HIV-positive patients with or without lipoatrophy or the HIV-negative study volunteers. These data, Dr. Roth explained, suggest that lipoatrophy may not necessarily be associated with an increased frequency of adipocyte apopotosis. “Other, possibly reversible, mechanisms may be at play,” he said.
Dr. Roth also pointed out that there have been a handful of studies indicating that lipoatrophy has improved in some patients, including those participating in clinical trials, upon switching from a potentially offending agent to one that is less likely to be associated with morphologic problems. “We know that lipodystrophy does improve, at least partially, when patients’ drug regimens are changed, at least in some cases,” he said.
An Introduction to Facial Fillers
The dermis is composed of several layers: the epidermis, the dermis, and the hypodermis. The epidermis is the outermost layer of skin. The dermis contains fibroblasts, hair follicles, sebaceous glands, apocrine glands, blood vessels, and nerves. The hypodermis, or subcutis, contains the adipocytes that help to give the dermis shape and thickness, along with larger blood vessels. It is the loss of fat in the subcutis that characterizes lipoatrophy.
Facial fillers involve either the dermis or the hypodermis (subcutis). Most facial fillers involve injections into the dermis, to thicken the skin. Some facial fillers involve injections into the subcutis to fill the space once held by adipocytes. Because these layers are only millimeters thick, expertise is required to ensure that the injections are performed safely and that the product is injected or inserted into the correct layer of the dermis.
The ideal filler, Dr. Roth argues, should replace deficient subcutaneous volume, be implantable with few risks, be affordable, and be temporary. “We don’t know what the future of lipoatrophy looks like, so we need to be able to prepare ourselves, in the event we need to remove a product from the face if the patient’s natural fat returns,” he explained. “The metabolic bases of lipodystrophy and lipoatrophy may be treatable.” Dr. Roth also stressed that the shape of the face changes over time, which can affect the placement and visibility of injected or implanted products. What’s more, long-term studies of “long-lasting” or “permanent” fillers are lacking. “Plus,” Dr. Roth cautioned, “some products may have undesirable outcomes, which means it’s very important for us to be able to remove it if necessary.”
There are a number of general differences between the various fillers being studied and used for HIV-associated lipoatrophy. For starters, fillers are either organic or synthetic. Organic fillers are naturally derived from humans, animals, or botanicals and are biodegradable. Synthetic fillers are generally not biodegradable. Facial fillers can also be classified as temporary, semi-permanent, or permanent. Temporary fillers are broken down and removed from the body over time, a process that can take from weeks to months. Semi-permanent fillers are not readily broken down and removed by the body, thus establishing permanence. However, they can be easily removed from the face, in the event of side effects or dissatisfaction with the results. Permanent fillers are much more difficult to remove from the face if problems or unhappiness with the results arise.
| II. Temporary Fillers | Top of page |

Figure 1. Autologous Fat TransplantDrawing of the technique for dermafat graft placement.
Source: Charles Herman, MD
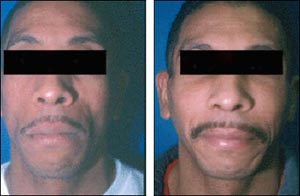
Figure 2. Autologous Fat Transplant
Before and one year after dermafat grafting.
Source: Charles Herman, MD
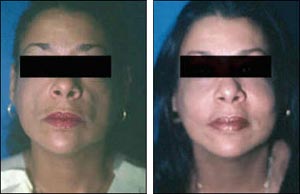
Figure 3. Autologous Fat Transplant
Before and one year after dermafat grafting.
Source: Charles Herman, MD
Autologous fat transplant
This procedure involves harvesting fat from one part of the body—such as the buttocks, hips, inner thighs, or abdomen—cleaning and filtering it, and injecting or surgically implanting it into another part of the body, such as the face. It is the most natural of all the facial fillers and can render results that are very natural in appearance. Plus, harvested fat can be frozen and stored if touch-ups are needed.
Dr. Charles Herman, a plastic and reconstructive surgeon affiliated with the Department of Plastic Surgery at Albert Einstein College of Medicine and Montefiore Medical Center, and Medical Director of Plastic Surgery at Pocono Health Systems in Pennsylvania, joined Dr. Roth in describing some of the cosmetic approaches being evaluated and utilized for the HIV-associated lipoatrophy. He described the use of dermal-fat grafting which, in contrast to fat injections, provides dermal substrate for vascularization of the fat component (see Figure 1).
According to Dr. Herman, “one advantage of this particular technique is the long-lasting result. Over three years, the correction is still very apparent. And, of course, another major advantage is that we’re using autologous material. It’s the patient’s own fat and dermis that is being used.” The dermal-fat grafting in patients with lipoatrophy, Dr. Herman explained, was pioneered and popularized by Dr. Berish Strauch, Chairman of Plastic Surgery at Montefiore Medical Center. Before-and-after images of two patients undergoing dermal-fat grafting are provided in Figures 2 and 3.
There are several disadvantages as well. For starters, subcutaneous fat can be difficult to harvest from other parts of the body, especially in HIV-infected patients with advanced lipoatrophy lacking in subcutaneous fat. “Subcutaneous fat can be traumatic to collect and traumatic to inject,” Dr. Roth said. “There’s a lot of post-operative swelling, with some people arguing that the corrective effect is almost entirely due to the post-operative swelling.” It should also be noted that injected fat can sometimes result in hypertrophy; this has been documented in patients who have fat harvested from enlarged dorsocervical fat pads (“buffalo humps”) for facial augmentation. “This is also a very expensive procedure,” Dr. Roth commented.
Collagen (Bovine: Zyderm and Zyblast; Human: CosmoDerm and CosmoPlast)
Collagen has been used for more than 25 years for cosmetic purposes in the United States, most notably as a filler for facial wrinkles. Because it has long been approved by the FDA for this purpose, it was one of the first products evaluated and used in HIV-positive people with facial lipoatrophy. Bovine collagen is derived from calf skins; human collagen is grown in vitro using human tissues. If bovine collagen is used, an allergy test is required before the product is injected into the face.
Both bovine collagen and human collagen are widely available and many plastic surgeons and dermatologists have experience using it, although not necessarily in HIV-infected patients with lipoatrophy. Disadvantages include the need for frequent touch-ups—collagen injections only last two to three months—and the possibility of allergic reaction or epidermal necrosis, resulting from pinched blood vessels in the face. “It’s rare, but it can happen,” Dr. Roth said. Repeated maintenance injections can increase the annual cost of therapy and large volumes may be needed to restore facial features in HIV-positive people with lipoatrophy.
Human cadaveric dermis (Cymetra, Dermalogen) and fascia (Fascian)
These products are derived from cadavers at the time of death. The dermis or muscle fascia is harvested, sterilized, tested, and processed. There is no need for allergy testing.
Cadaveric dermis and fascia are FDA approved—although not for HIV-associated lipoatrophy—and are readily available. Anecdotal reports suggest that it results in impressive filling of hollows in the face, at least initially. Drawbacks include the need of a large-gauge needle for injection. It can also be very expensive, given the frequent need for touch-ups.
Hyaluronic Acid (Restylane, Restylane SubQ, Perlane, Hylaform)
Hyaluronic acid is naturally found in human connective tissues. These four brands are synthetic versions of hyaluronic acid and have been designed to prevent rapid breakdown by the body. Injections of hyaluronic acid have been shown to last six to 12 months in HIV-positive individuals with lipoatrophy. Over the long term, they remain cheaper than many other products, given that fewer touch-ups are needed. Hyaluronic acid fillers can also be easily reversed by injecting hyaluronidase (Vitrase). “Post-injection recovery can be slightly uncomfortable,” Dr. Roth said. “Large volumes are needed for patients with moderate-to-severe facial lipoatrophy.”
Dr. Roth is most interested in a new form of hyaluronic acid called Restylane SubQ. Whereas standard versions of hyaluronic acid are intended for injection into the dermis, Restylane SubQ has a large particle size and can be used to fill the subcutis. “This is exactly what we want out of these fillers,” he said. “Unfortunately, it’s not readily available in the United States, at least not yet.”
Only standard Restylane is approved for use in the United States. Restylane SubQ, Perlane, and Hylaform are available in Europe and South America.
Calcium hydroxylapatite (Radiesse, Radiance)
These products contain synthetic calcium hydroxylapatite, a natural substance found in bones and teeth. It is primarily used in the reconstruction of bony structures. When it is injected into the dermis layer of skin, natural collagen forms around the calcium hydroxylapatite, providing long-term, natural-looking fullness.
Calcium hydroxylapatite is approved by the FDA for various uses in the United States, including orthopedic and reconstructive surgery and in dentistry and has a good safety record. Even though it is considered to be a temporary filler, it appears to have a longer lasting effect than most other temporary fillers. It can be very expensive. It can also cause nodules at the injection site—they can be felt but not usually seen—in some patients.
“The injection technique is relatively simple,” explained Dr. Joseph Eviatar, Assistant Professor of Ophthalmology ant NYU Medical School, who briefly described his experiences and observations as an investigator of a study evaluating calcium hydroxylapatite in HIV-infected patients with lipoatrophy. “What you inject is pretty much what you get. In other words, if you inject a certain volume, that’s what you’ll see. You have to account for a little bit of swelling, but you can inject it where you’d like it and the product pretty much stays there. It’s a soft and malleable product. The material is injected subcutaneously. Placing the material in the subcutaneous plane is most physiologic and gives the most natural result."
Dr. Stacey Silvers, Associate Adjunct at Beth Israel Medical Center and St. Vincent’s Medical Center—and another calcium hydroxylapatite investigator—concurred with Dr. Eviatar. “So far, we’ve been very happy with this product in the study we’ve been participating in. The patients have also been very pleased with it so far. In European studies, it has been suggested that hydroxylapatite is restorative for two to five years. We haven’t seen this—we’re seeing results lasting approximately a year to a year and a half—but it’s one of the longest lasting fillers I’ve seen.”
Sample before-and-after pictures are provided in Figures 4 and 5.
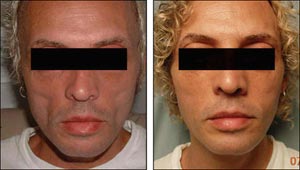
Figure 4. Calcium hydroxylapatite (Radiesse)
Before and one year after Radiesse injections.
Source: Joseph Eviatar, MD

Figure 5. Calcium hydroxylapatite (Radiesse)
Before and six months after Radiesse injections.
Source: Stacey Silvers, MD
Poly-l-lactic acid (Sculptra, New-Fill)
Sculptra is an injectable poly-L-lactic acid (PLA) implant in the form of a sterile lyophilized cake. It contains microparticles of poly-L-lactic acid, a biodegradable synthetic polymer from the alpha-hydroxy-acid family. Sculptra, branded as New-Fill in more than 30 countries outside of the United States, requires reconstitution prior to injection to form a sterile non-pyrogenic suspension.
Even though it is synthetic, it is eventually broken down and removed by the body, meaning that its effects are temporary. It is the only facial filler to be approved by the FDA for the reconstructive management of HIV-associated facial lipoatrophy. The number of sessions will depend on the severity of each case.
Poly-L-lactic acid is injected into the deep dermis or the subcutaneous layer. The two basic techniques for injection are: tunneling (also called threading) and depot. Tunneling, used to deposit poly-L-lactic acid deep in the dermis, is used for injections of the lower face (e.g., cheeks and nasolabial folds). Depot, used to deposit poly-L-lactic acid between the periosteum and muscle, is used for injection of the upper zygoma, temples, and periorbits. Injections are typically followed by a thorough 20 minute massage to ensure proper placement and to reduce nodules, as well as icing to limit swelling.
The most notable advantage of poly-L-lactic acid is that its safety and effectiveness in the management of HIV-associated lipoatrophy have been evaluated by the FDA. Even though it is considered to be a temporary filler, it appears to have a longer lasting effect than most other temporary fillers. Repeat injections, within a year or two after an initial treatment, are likely necessary. Dr. Gervais Fréchette, a New York City-based clinician who serves as an investigator for studies evaluating poly-L-lactic acid in HIV-infected patients with lipoatrophy, commented that he has some patients who have gone four years without the need for touch-up injections.
See Figure 6 for before-and-after photographs involving a patient receiving Sculptra.
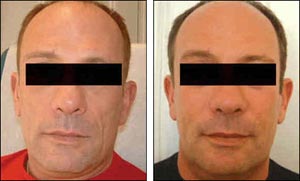
Figure 6.Poly-L-Lactic Acid (Sculptra)
Before and after Sculptra infections.This patient received four treatments in total.
Source: Gervais Fréchette, MD
“I’ve been very pleased with this product,” explained Dr. Fréchette. “As with all facial fillers, adverse events are possible. Bleeding and bruising are possible. Bumps and lumps are definitely a problem that we have to address. If too much product is injected in one area, this can cause problems as well. Problems can also arise if the session is done too quickly. Nodules can also happen spontaneously, even weeks or months after the procedure is done.” However, he reiterated, the risk of nodules can be greatly reduced with thorough post-injection facial massage.
The cost of poly-L-lactic acid can be high, although the manufacturer of Sculptra (Dermik Laboratories) has implemented a sliding-scale fee for the product based on an individual’s income (it is provided for free in some cases). Multiple sessions can be cumbersome and increase the cost of treatment, although Dr. Frechette argues that is the most cost-effective filler available.
Many medical doctors around the United States are now providing access to poly-L-lactic acid for the management of facial lipoatrophy in HIV-infected patients. Dermik Laboratories has developed a searchable database to help HIV-positive individuals and primary care providers locate medical doctors offering this service and is accessible at http://www1.sculptra.com/US/Locator.do.
III. Semi-permanent and Permanent Fillers
All of the semi-permanent and permanent fillers reviewed here are synthetic products and are not readily broken down and removed by the body. In other words, they are supposed to have lasting effects upon being injected or inserted into the face. Semi-permanent fillers can be removed, usually through a minor procedure, in the event of side effects or dissatisfaction with the results. Permanent fillers cannot be easily removed.
Silicone
Silicone comes in solid formulations and liquid formulations. Solid silicone, which can be used for cheek and chin implants, “is excellent for correcting bony defects in patients who don’t have prominent cheekbones,” Dr. Roth commented. “It’s very difficult to inject a lot of filling material into somebody who doesn’t have a bony structure that will support it. Some people have a prominent zygomatic arch, whereas others don’t. People who have a very flat face, or who rely completely on their bucal fat pads to give their face dimension, are most likely to benefit from solid implants, used in combination with filling material.”
Dr. Silvers noted that she has performed more than 300 cheek implants for lipoatrophy patients, some in conjunction with injectable fillers. “Most patients,” she commented, “have been very pleased with the results.” Before-and-after photographs, involving a patient who received cheek implants in conjunction with Radiesse injections, are provided in Figure 7.
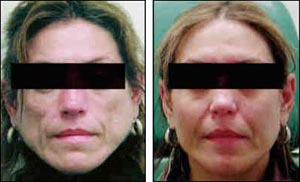
Figure 7.Silicone Implants and Injectable Filler
Before and after Silicone implants, coupled with Radiesse injections.
Source: Stacey Silvers, MD
Silicone oil is another possibility, although it is only approved in the United States for use as postoperative retinal tamponade during vitreoretinal surgery. It is still being investigated for facial augmentation. However, anecdotal reporting suggests that it is safe and effective when injected in very small volumes (microdroplets). A chief disadvantage is that it cannot be removed. It can be toxic and damaging to the face if too much is injected and can migrate—usually down toward the jawline—causing new cheeks to become jowls. “I think it’s a terrible product for lipoatrophy, mostly because it is permanent,” Dr. Roth remarked. “Once it’s in, it’s in… there’s nothing you can do about it.”
Polymethylmethacrylate (PMMA; Artecoll, Artefill)
PMMA is best known for its use in manufacturing hard contact lenses and Plexiglass. These products contain small particles (microspheres) of PMMA that are surrounded by bovine collagen. Approximately three months after it is injected, the bovine collagen is broken down and removed from the body, but is replaced by natural collagen. The PMMA molecules and the surrounding collagen persist indefinitely. The FDA has indicated that is safe and effective for the correction of facial wrinkles, lines, and furrows, but has not yet officially approved the product.
PMMA is a semi-permanent filler and can be removed in the event of side effects or dissatisfaction with the end result. Side effects are possible, especially if large amounts of PMMA are used. And it can sometimes be felt, but rarely seen, under the skin.
Expanded Polytetrafluoroethylene (ePTFE) Implants (Gore-Tex, Gore SAM, SoftForm)
These solid implants require minor surgery, via a small incision, under local anesthesia. They have been used for many years to help restore deep facial defects and may be useful for HIV-associated lipoatrophy in terms of filling large, sunken areas. Dr. Roth indicated that it may be best to use ePTFE implants with other fillers, particularly those that trigger collagen production in the dermis.
“This product really isn’t used much anymore,” Dr. Roth explained. “It’s nice, but it does come with the possibility of post-operative complications, such as infection and swelling, and can result in fibrosis around the implant. Even though it can technically be removed, the fibrosis can make this difficult.”
Polyalkylamide (Bio-Alcamid)
Polyalkylamide is a synthetic product that can be injected using a high volume, making it a possible option for HIV-positive individuals with severe lipoatrophy. There is very little experience testing or using Bio-Alcamid in the United States. However, it has been used in Europe for cosmetic and reconstructive purposes, with good results, and is the product of choice at a clinic in Tijuana that has yielded a lot of encouraging before-and-after photographs. Polymekon-USA, the American division of the Italian-based Polymekon that manufactures Bio-Alcamid, is planning clinical trials in the United States.
“The remarkable thing about this product is that it’s a permanent product, given that it is encapsulated in collagen in the subcutis,” Dr. Roth explained. “It’s removable by incision and expression of the material. It doesn’t appear to matter if it’s a week later, a year, later, or ten years later; it seems to be an extremely stable product. It definitely meets an important economic criterion: permanent correction. But it is reversible if lipoatrophy is reversed.”
Before-and-after photographs, of two patients who received Bio-Alcamid, are provided in Figures 8 and 9.
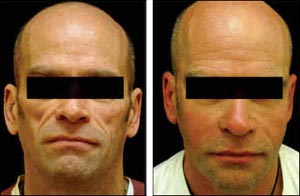
Figure 8.Polyalkylamide (Bio-Alcamid)
Before and after Bio-Alcamid injections.
Source: Clinic'estetica

Figure 9.Polyalkylamide (Bio-Alcamid)
Before and approximately six weeks after Bio-Alcamid injections. A high volume of Bio-Alcamid was needed in this patient. Each temple area required 6 ccs of filler.
Source: Clinic'estetica
Dr. Roth also explained that a very large volume of polyalkylamide can be injected during one session. “In patients with severe lipoatrophy, large volumes of the product will likely be needed, which appears to be feasible with this product.”
A disadvantage, of course, is that it is not yet available in the United States. Plus, there has been very little sound, scientific data supporting its safety or effectiveness.
Issues of Cost
The truth of the matter is that few private health insurance companies are routinely covering the costs of these products, even for poly-L-lactic acid, which has been approved for use in HIV-positive people with facial lipoatrophy. And at this point in time, neither Medicaid nor ADAP are covering the costs of these procedures. In turn, these costs are a huge consideration for almost all HIV-infected patients wishing to undergo restorative treatment.
As estimated by Dr. Roth, the average cost associated with poly-L-lactic acid treatment is $1,500 per office visit, which includes the price of the product and costs associated with the procedure itself. “Typically two to sex visits are required, which means that this can be a costly option,” he said.
The cost of silicone oil injection is variable. “It depends on who is doing it,” Dr. Roth said. “It depends on the technique. It depends on how much silicone is used. It depends on how many sessions are needed. And even if it turns out to be one of the cheapest products, it’s not a procedure that I recommend… ever.”
Collagen can also be obtained at relatively low cost. “But, again, it’s not an ideal material because you can only put it into the dermis. We really want to focus on materials that can be inserted into the subcutis.”
Restylane SubQ is not yet priced in the United States, but Dr. Roth estimates that a cost of $3,000 will likely apply. “This figure applies for the first year of therapy,” he said. “Touch-ups, on an annual basis, using this product may be less.”
Autologous fat transplants, Dr. Roth reckons, are likely to be the most expensive. Gortex implants are also likely to be expensive, given the surgical precision required, along with the fact that it’s best used in combination with another filler. The price of Radiesse has come down recently. “It used to be available to doctors at approximately twice the price it’s available now, so this is encouraging,” Dr. Roth commented. As for Bio-Alcamid, the cost of international travel to secure access to this product can be an economic barrier.
Of course, some patients have had luck securing health insurance reimbursement or payment for these facial fillers. There have been anecdotal reports of clinicians being able to convince insurance companies that their patients require a recommended facial filler to restore their facial features lost to antiretroviral treatment, not simply for cosmetic purposes. This is very similar to the successful argument made by many women with breast cancer, who required a mastectomy, that breast reconstruction be a covered expense.
It is with hope that the HIV medical establishment will universally accept these facial fillers as necessary restorative therapies for HIV-positive people with lipoatrophy. This may eventually result in routine coverage of these products and procedures by private and public health insurers. Unfortunately, for many HIV-positive people with facial lipoatrophy, these products—and the expertise needed to inject these products safely and effectively—remains an out-of-pocket and costly expense.
| References | Top of page |
