The impact that HIV has on the pathogenesis of tuberculosis (TB) is clear. It is one of the most important risk factors associated with an increased risk of latent TB infection (LTBI) progressing to active TB disease. HIV-infected people have an annual risk of 5% to 15% of developing active TB once infected (Rieder, 1999). TB is the most common opportunistic infection in people living with HIV worldwide. It is also the most common cause of death in HIV-positive adults living in developing countries, despite being a preventable and treatable disease (Corbett, 2003).
Dr. Sonal Munsiff began her April 2006 PRN lecture describing the global epidemiology of TB and HIV coinfection and emphasized its relevance to New York City’s large immigrant population. Following a discussion of the current status of TB and HIV coinfected patients in New York City, she focused on the diagnosis and treatment challenges in these patients.
The estimated incidence of TB remains high in both Asia and Africa, with the highest estimated incidence in sub-Saharan Africa (300 new cases per 100,000 population). “The main reason for the markedly high rates of TB in sub-Saharan Africa is the high prevalence of HIV infection,” Dr. Munsiff pointed out. “In fact, more than half of TB cases in many sub-Saharan African countries are in HIV-infected persons. The AIDS epidemic has caused marked acceleration of the TB epidemic worldwide and in sub-Saharan Africa, in particular” (see Figure 1).
The Stop TB Partnership recently released a ten year plan to address the global TB epidemic (Stop TB Partnership, 2006). While this plan aims to reduce the global burden of TB, Dr. Munsiff noted that even if all aspects of the plan are implemented and fully funded, a decrease in TB incidence in African countries with high HIV prevalence in the next ten years is probably unlikely based on current trends.
While TB and HIV co-infection remains a major public health problem in many parts of the world, the number of TB cases coinfected with HIV in the United States has been decreasing. The number of TB cases has been declining since 1992, and the prevalence of TB and HIV coinfection has reached a somewhat steady state. According to the U.S. Centers for Disease Control and Prevention (CDC), approximately 10% of all TB cases in the US are also co-infected with HIV (CDC, 2004).
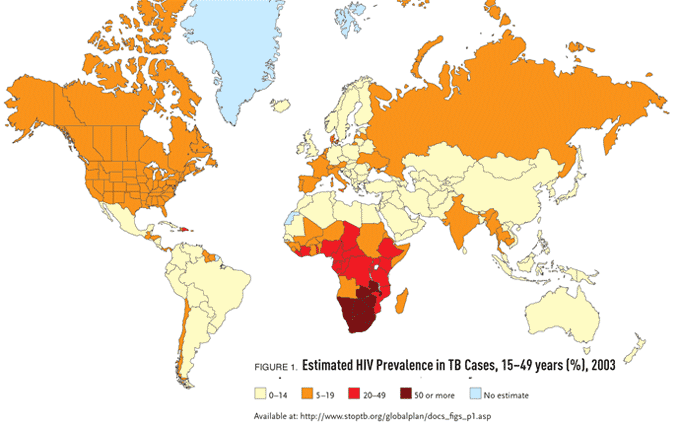
Figure 1.Estimated HIV Prevalence in TB Cases, 15–49 years (%), 2003
| Table 1. Demographic and Clinical Characteristics of TB patients by HIV status in New York City, 2000–20051 | ||||||
| Variable | HIV-infected patients (n=1,113) | HIV-uninfected patients (n=3,580) | OR | P value | ||
| N | % | N | % | |||
| Age | ||||||
| 0 –18 | 8 | 0.7 | 264 | 7.4 | 0.09 | <.0001 |
| 19–44 | 671 | 60.3 | 2,085 | 58.2 | 1.09 | .226 |
| 45–65 | 412 | 37.0 | 881 | 24.6 | 1.80 | <.0001 |
| 65 and over | 22 | 2.0 | 350 | 9.8 | 0.19 | <.0001 |
| US-born | 707 | 63.5 | 962 | 26.9 | 4.74 | <.0001 |
| Homeless | 203 | 18.2 | 167 | 4.7 | 4.56 | <.0001 |
| History of Substance Abuse | 593 | 53.3 | 640 | 17.9 | 5.24 | <.0001 |
| Race/ Ethnicity | ||||||
| Non-Hispanic White | 62 | 5.6 | 264 | 7.4 | 0.74 | .039 |
| Non-Hispanic Black | 708 | 63.6 | 1,046 | 29.2 | 4.23 | <.0001 |
| Hispanic | 308 | 27.7 | 1,226 | 34.2 | 0.73 | <.0001 |
| Asian | 34 | 3.1 | 1,030 | 28.8 | 0.08 | <.0001 |
| Culture Positive | 879 | 79.0 | 2,769 | 77.4 | 1.10 | .254 |
| Respiratory Smear Positive | 522 | 47.0 | 1,549 | 43.3 | 1.16 | .033 |
| Tuberculin Skin Test Positive2 | 313 | 52.8 | 1,929 | 80.0 | 0.28 | <.0001 |
| Site of Disease | ||||||
| Pulmonary Only | 632 | 56.8 | 2,482 | 69.3 | 0.58 | <.0001 |
| Extrapulmonary Only | 202 | 18.2 | 808 | 22.6 | 0.76 | .002 |
| Both | 279 | 25.1 | 290 | 8.1 | 3.80 | <.0001 |
| Chest Radiograph Status | ||||||
| Normal | 261 | 23.5 | 587 | 16.4 | 1.56 | <.0001 |
| Abnormal/Cavitary3 | 74 | 11.7 | 635 | 25.6 | 0.39 | <.0001 |
| Abnormal/Non-cavitary2 | 476 | 75.3 | 1,776 | 71.6 | 1.21 | .059 |
| Multidrug-resistant TB | 33 | 3.0 | 83 | 2.3 | 1.29 | .225 |
| Other Drug Resistance | 99 | 8.9 | 363 | 10.1 | 0.87 | .223 |
1 hiv-status was unknown in 2,079 patients. 2 Among those that were tst tested. 3 Among pulmonary tb patients only. |
||||||
| I. TB and HIV Coinfection in New York City | Top of page |
In New York City, we had a very large TB epidemic,” Dr. Munsiff recounted. “Case rates reached about 52 per 100,000 at the height of our recent epidemic in 1992, at which time, at least a third of all patients with TB were HIV-infected. We think it was closer to 40% because there was a lot of underreporting and underestimation.” Over the past 15 years, the percentage of TB cases who are also HIV infected has dropped and now hovers around 15% to 18% of all cases (New York City Department of Health and Mental Hygiene, 2005). While the percentage of TB patients with an unknown HIV status has also decreased from 51% in 1992, it still remains quite high at 28% in 2005. The Bureau of Tuberculosis Control (BTBC) is trying to increase counseling and testing of coinfected patients by offering rapid and conventional HIV testing at all its TB chest centers. For known HIV-infected TB cases, the BTBC has also increased efforts to get high-risk contacts tested for HIV infection.
Data from the Bureau of HIV/AIDS Prevention and Control indicate that in 2000, TB was second to only Pneumocystis pneumonia (PCP) as the most common opportunistic infection in HIV/AIDS patients in New York City.
In New York City, the prevalence of HIV infection among TB patients is higher in men than women. Over the past 5 years, 18% of all male TB cases were HIV-infected, compared to 11% of all female TB cases. Dr. Munsiff explained, “It used to be that the disparity was much greater with almost half the US-born men having HIV infection versus women but the disparity has gone down.” A demographic review of the past six years of TB cases in NYC by HIV status shows that 97% of HIV-infected TB cases are between 19 and 65 years of age, and 64% are US-born (see Table 1).
Compared to the HIV-negative TB patients, HIV-infected TB cases are more likely to be homeless and have history of substance abuse, emphasizing that these patients are often disenfranchised from the community. Dr. Munsiff explained, “It’s a population that’s very hard to reach. Part of the reason why we continue to see a lot of TB is that the patients are not staying in care and receiving treatment, especially for latent TB infection, which could prevent this opportunistic infection.” BTBC’s Homeless Services Unit continues to assess barriers to TB screening at homeless shelters and implement methods to improve screening and treatment of LTBI. They are working to improve programs to conduct contact investigations of infectious cases in the Department of Homeless Services (DHS) shelters, and provide more directly observed therapy (DOT) for LTBI. They also offer TB education for medical providers working in DHS shelters.
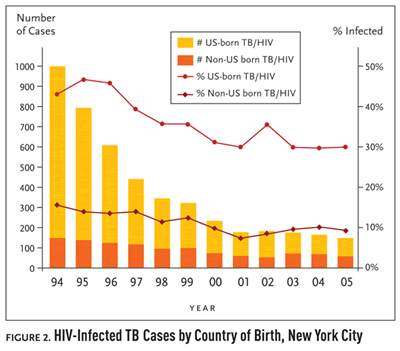
Figure 2. HIV-Infected TB Cases by Country of Birth, New York City
While US-born TB cases have decreased by 90% since 1994, Dr. Munsiff went on to say “the percentage of US-born people with TB who have HIV infection really hasn’t changed that much in the past years.” The percentage of US-born TB cases infected with HIV has only dropped 13%, from 43% in 1994 to 30% in 2005 (see Figure 2). “A lot of this reflects the extensive transmission that occurred in the late eighties and early nineties in New York,” Dr. Munsiff said. “There is also ongoing transmission among HIV-infected persons in some congregate residential settings for AIDS patients now. HIV-infected persons living in these facilities are at a greater risk for becoming infected with TB, and onceinfected, are more likely to develop active TB disease due to theirimmunocompromised state.”
There is also a high risk of re-infection and developing active TB once exposed, even if LTBI treatment was completed in the past (CDC, 1998). TB diagnostic tests, such as the tuberculin skin test, do not always indicate if infection is present in immunosuppressed individuals. The BTBC conducts contact investigations and screenings in congregate facilities where TB exposures have occurred. In 2004 and 2005, nineteen such investigations were conducted. Despite these efforts, there have been several outbreaks in residential facilities for HIV-infected persons where extensive transmission occurred. (See Sidebar: Evidence of Recent Transmission in TB and HIV-infected Populations).
The evidence of recent transmission in these facilities underscores the problem of putting severely immunocompromised individuals in congregate settings. Molecular epidemiologic studies indicate recent transmission among individuals who have TB due to clustered strains. Our data have shown that 60% of HIV-infected TB cases in NYC from 2001 to 2005 were genotypically clustered, compared to 40% of HIV-negative cases. While some clustered TB strains do not indicate recent transmission because they are endemic in NYC and may have been acquired several years earlier, the difference between these two groups is significant and is consistent with evidence of ongoing TB transmission in this population (Driver, 2006).
A review of the clinical characteristics of TB patients who also have HIV infection indicated that most (79%) were culture-positive for TB; 21% were clinically diagnosed. HIV-infected TB cases were more likely to have both pulmonary and extrapulmonary disease compared to HIV-negative TB cases, and among HIV-infected extrapulmonary TB cases, there was a higher percentage of meningeal as well as miliary or disseminated TB (see Table 1). HIV-infected TB cases were also more likely to have a normal chest radiograph and less likely to have a cavitary chest radiograph compared to HIV-negative TB cases. TB cases with unknown HIV status were not included in this analysis.
The prevalence of drug resistance did not differ substantially between HIV-positive and HIV-negative TB cases. Dr. Munsiff stated, “New York City has a moderately high prevalence of drug resistance, which is why an empiric four-drug treatment regimen of isoniazid, rifampin (or rifabutin), pyrazinamide and ethambutol is still the standard treatment until susceptibility results become available.”
Clinical outcomes, however, did differ significantly for HIV-infected TB cases (see Table 2). From 2000 to 2005, 18% of all HIV-infected TB cases died during the course of treatment, compared to 3% of all HIV-negative TB cases. Dr. Munsiff noted, “If they have not died before their TB treatment is over, most remain in care and finish treatment. Overall default rates are very low. And whether the cause of death is from TB is very hard to tease out.” However, of all culture-positive HIV-infected TB cases who died before completing treatment, approximately 50% were still culture positive within thirty days of death. TB likely contributed to death in most of these cases.
Additionally, Dr. Munsiff showed data from two cohorts of HIV-infected TB patients in NYC, one group from 1996 (pre-highly active antiretroviral therapy [HAART]) and another from 2005 (post-HAART). In both groups, approximately 70% of patients knew their HIV positive status at the time of TB diagnosis. Dr. Munsiff indicated, “TB diagnosis is not the time when the initial diagnosis of HIV was made in most of the coinfected patients. They were already known to have HIV infections, but most of them were not in care.” The median CD4+ count was less than 200 cells/mm3 for both groups. Therefore, it is likely that most of these patients were eligible for HAART, and yet, only 18% of the HIV-infected TB cases in 1996 were on a protease inhibitor (PI). In 2005, only 33% of the HIV-infected TB cases were on some type of antiretroviral treatment. These data further identify the gaps in the diagnosis and treatment of TB\ patients with HIV infection.
| Table 2. Treatment Outcomes of TB patients by HIV status in New York City, 2000–2005* | Treatment Outcomes | HIV-infected patients (n=1,113) | HIV-uninfected patients (n=3,580) | or | P value | |
| N | % | N | % | |||
| Completed treatment | 748 | 67.2 | 3,021 | 84.3 | 0.38 | <.0001 |
| Died prior to treatment completion | 201 | 18.1 | 122 | 3.4 | 6.25 | <.0001 |
| Still on treatment as of April 18, 2006 | 106 | 9.5 | 282 | 7.9 | 1.23 | .081 |
| Lost to follow up | 38 | 3.4 | 67 | 1.9 | 1.85 | .002 |
| Refused treatment | 10 | 0.9 | 35 | 1.0 | 0.92 | .813 |
| Moved out of New York City | 10 | 0.9 | 53 | 1.5 | 0.60 | .141 |
| * HIV-status was unknown in 2,079 patients. | ||||||
In March 2006, the BTBC launched a TB Awareness Campaign on World TB Day. The campaign, “Moving Toward a Tuberculosis-Free New York City,” combines local media advertising and partnership efforts with medical providers and community-based organizations to target communities in NYC with high rates of TB infection and disease. Specific outreach is ongoing to HIV providers and AIDS clinics in NYC to ensure testing and treatment for LTBI. While the BTBC offers directly observed therapy (DOT) to all active TB cases on treatment, it is also working to expand DOT for HIV-infected persons on LTBI treatment as well.
| II. Update on the Diagnosis and Management of TB/HIV Coinfected Patients | Top of page |
Nucleic Acid Amplification Testing to Improve Diagnosis of TB
“The diagnosis of TB remains difficult, whether you have HIV infection or you don’t,” Dr. Munsiff asserted. “The smear for acid-fast-bacilli (AFB) is still used in most parts of the world to diagnose patients with TB and it has many limitations. About a decade ago, a nucleic acid amplification (NAA) test became available that allows you to essentially make a rapid confirmation of TB. These tests are not used as much as they could be.” Dr. Munsiff discussed several aspects of how the NAA test can be used to diagnose tuberculosis. While traditional laboratory culture methods require 1-8 weeks, direct molecular methods using NAA can detect genetic material of Mycobacterium tuberculosis complex directly from clinical specimens within three to five hours.
Two FDA approved NAA assays are available: the Gen-Probe AMPLIFIEDTM Mycobacterium tuberculosis Direct (MTD) Test for AFB smear-positive or -negative respiratory specimens and the Roche Amplicor® Mycobacterium tuberculosis (MTB) Test for smear-positive specimens only. Most clinical experience with the tests is from patients with moderate to high clinical suspicion of TB. The sensitivities of these tests range from 95% to 100% in AFB smear-positive and 60% to 80% in smear-negative respiratory specimens from patients with a high clinical suspicion of TB (CDC, 2000).
“The tests are not only useful for all patients who have a positive AFB smear from respiratory specimens but they are also appropriate to use in patients who have negative AFB smears, but in whom your clinical suspicion of TB is high and who have had less than seven days of TB treatment,” Dr. Munsiff noted. “You don’t want to use this test in every patient in isolation to rule out TB since in most facilities, only one of 8 or 9 patients placed in airborne isolation is confirmed to have active TB. However, if the patient will be starting treatment for TB because that is one of the leading diagnoses – even if the smear is negative – then an NAA test is very useful because a rapid confirmation allows discontinuation of empiric treatment for other diseases.”
Two laboratories – the NYC Public Health Laboratory and the Wadsworth Center of the New York State Department of Health (NYS DOH) – provide the MTD test at no charge when TB is newly suspected. Yet only about half of all patients with pulmonary AFB smear-positive specimens are actually having an NAA done on their specimen. NAA testing has the potential to profoundly affect treatment decisions, as well as keeping costs down through reduced hospitalization and patient isolation. Dr. Munsiff explained, “Many AFB smear-positive patients in New York City do not have TB, so it can also help you rule out TB very quickly, even in an AFB smear-positive patient.”
A positive NAA test in conjunction with an AFB-positive smear is highly predictive of TB disease. However, the results of NAA tests are considered preliminary; mycobacterial culture is still needed for species identification/confirmation and for drug susceptibility testing.
A negative NAA with an AFB-positive smear indicates that the AFB is probably non-tuberculous mycobacteria. In the absence of clinical symptoms, these results may lead the physician to discontinue isolation and anti-TB treatment; moreover, there may be no need to evaluate people exposed to the individual for TB infection. The diagnosis in such a case will depend on the overall clinical evaluation, medical judgment, and repeat NAA or other laboratory methods.
Recent New York State regulations require laboratories to perform rapid diagnostic tests using NAA methods on AFB smear-positive initial sputa or respiratory specimens (New York City Department of Health and Mental Hygiene, 2006). Thus, these results should be available to the clinician for all such patients without making a separate request. Clinicians will still need to order NAA tests for smear negative specimens.
The Problem of Rifamycins and Drug-Drug Interactions in TB and HIV Coinfected Patients
“TB is rapidly fatal, especially in HIV-infected persons,” Dr. Munsiff stressed. “Effective treatment rapidly reduces the number of organisms and renders the person noninfectious. It sterilizes lesions to prevent relapse. Multi-drug treatment prevents acquired drug resistance. Single-drug treatment should never be given for active TB. Treatment essentially is curative and rifampin, rifabutin or rifapentine, which I refer to as rifamycins, are essential in TB treatment.”
Treatment of TB in the presence of HIV infection is complicated by drug-drug interactions between rifamycins, and PIs and non-nucleoside reverse transcriptase inhibitors (NNRTIs). Both PIs and NNRTIs are metabolized by hepatic CYP3A, specifically the CYP3A4 isoenzyme. Rifamycins are inducers of the CYP3A family of enzymes, which includes the CYP3A4 isozyme. Maximal drug levels (represented by Cmax) or total drug exposure over time (represented by AUC, area under the concentration-time curve) of antiretroviral agents may be reduced when these drugs are coadministered with rifamycins, reducing the efficacy of HAART regimens.
Criteria for requesting NAA tests:
High clinical suspicion of tb, previously untreated or <7 days of treatment*
Respiratory specimen, or Non-respiratory specimens (request from the lab on a case by case basis if clinical suspicion is high)
* Since the naa test can amplify rrna from both viable and nonviable organisms, and therefore may detect nonviable tubercle bacilli expelled by an individual being treated for tb, the test result may be positive even though the treatment has decreased the likelihood that the tb is infectious.
Regimens that include rifampin are much shorter duration (6 to 9 months vs. 18 to 24 months) and have faster sputum conversion rates, higher cure rates, and lower relapse rates than regimens that do not include rifampin. Regimens that have used only two months of rifampin (or rifamycin) have been shown to have higher relapse rates, particularly for HIV-infected patients (Munsiff, 2006). Rifabutin is not FDA approved for the treatment of TB, but it is used by many clinicians, and there is a record of now almost a decade of fairly extensive use. Rifabutin may be substituted for rifampin, either initially or during the continuation phase of treatment (the usual dose of rifabutin is 300 mg/day). Rifabutin should be substituted at least two weeks before the planned initiation of a PI or an NNRTI to allow for the resolution of the effect of rifampin on CYP3A.
For drug-sensitive TB, several rifamycin-containing anti-TB regimens can be safely administered with effective antiretroviral therapy. None of the nucleoside reverse transcriptase inhibitors (NRTIs) have any drug interactions with rifamycins that are of clinical significance. Rifampin and rifabutin are the preferred rifamycins for HIV-infected patients taking PIs or NNRTIs.
Rifamycins and NNRTIs
Some NNRTIs can be used with rifampin. Rifampin modestly decreases efavirenz (Sustiva, Atripla) exposure. Therefore, it is probably safe to use rifampin concomitant by prescribing a slightly higher dose of efavirenz, though some investigators report that there appears to be no need to increase the efavirenz dose when administered with rifampin This regimen is also useful in resource-poor countries where rifabutin is generally not available.
Nevirapine exposure is also reduced by rifampin. Several small observational studies have shown a favorable clinical response for patients receiving rifampin and nevirapine. Coadministration of nevirapine and rifampin may be particularly useful in resource-poor countries for pregnant patients; efavirenz cannot be used in pregnancy and use of a PI-based regimen is limited because rifabutin is generally not available. If used under these circumstances, close clinical and virologic monitoring should be performed. However, nevirapine is contraindicated in women with CD4+ counts above 250 cells/mm3 because of an increased risk of severe hepatotoxicity. In such women who are pregnant a HAART regimen will be difficult to administer during TB treatment in areas where rifabutin is not available. Nevirapine exposure is only slightly decreased by rifabutin, and nevirapine also slightly decreases rifabutin exposure. Therefore, nevirapine can be used with rifabutin, both at their usual doses.
While rifabutin can also be used with both efavirenz and nevirapine, another NNRTI, delavirdine, should not be used with either rifampin or rifabutin because both rifamycins greatly diminish blood concentrations of delavirdine.
Rifamycins and PIs
While rifampin cannot be used with any of the PIs, rifabutin can be used with regimens containing a single PI—except saquinavir (Invirase) alone--with some dose adjustments. Rifabutin can also be used with the lopinavir/ritonavir (Kaletra), fosamprenavir (Lexiva)/ritonavir, darunavir (Prezista)/ritonavir, or tipranavir (Aptivus)/ritonavir combinations; all are FDA-approved combinations. Rifabutin should not be used with ritonavir alone because of a high rate of adverse effects. For any boosted regimen containing ritonavir, the dose of rifabutin should be reduced.
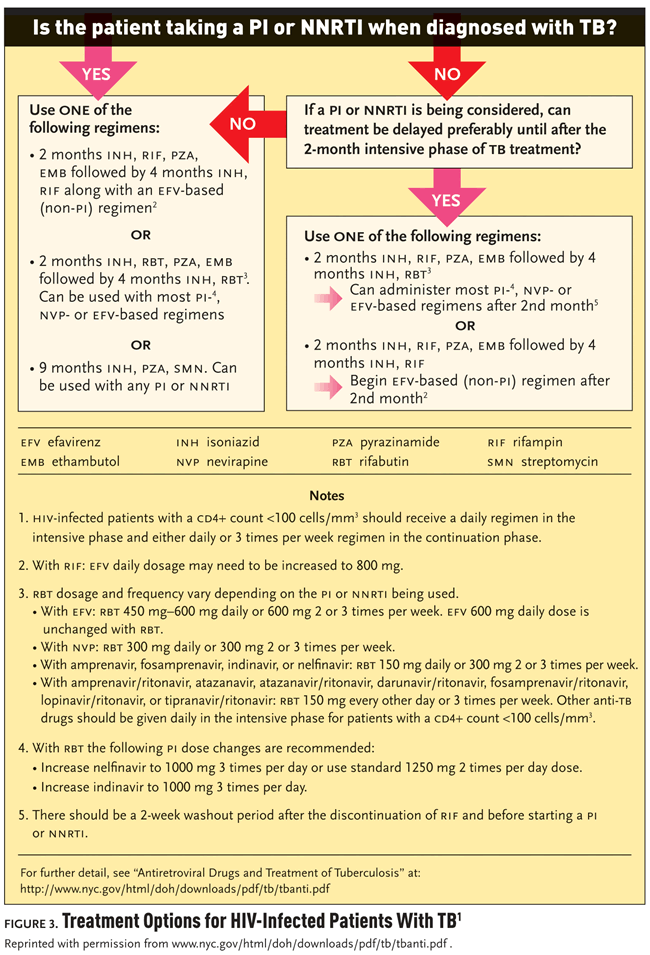
Choosing the Right Treatment
Dr. Munsiff has designed a flow chart to guide physicians in treating TB and HIV coinfection (see Figure 3). It is important to know if the patient is taking antiretroviral treatment at the time TB treatment is being initiated, since that will affect treatment options. If the patient is on antiretroviral treatment, Dr. Munsiff stated, “Most patients get started on isoniazid, pyrazinamide, and ethambutol, along with either rifampin or rifabutin, depending on the HAART regimen. If you have rifamycin resistance, which is uncommon, you can use whichever antiretroviral regimen you prefer.” The last option is to give a non-rifampin-based regimen which includes the injectable agent streptomycin for nine months. While there are no drug interactions, few clinicians and patients opt for this treatment due to the need for injections for nine months.
If the patient is not on a PI- or NNRTI-based regimen when the TB is diagnosed, there are several options. It is important to initiate TB treatment first. Antiretroviral treatment does not have to start immediately; the clinician needs to make a decision on when it is appropriate to begin antiretroviral treatment (Dean, 2002; Moreno, 2005). Dr. Munsiff explained, “Some data suggest that waiting at least a few weeks before initiating therapy may be appropriate for some patients and if it can be delayed for two months, it may be more desirable. Immune reconstitution inflammatory syndrome (IRIS) seems to be less common after at least several weeks have passed. There are fewer drugs to use after two months, since in most patients with sensitive TB, the number of TB drugs can be decreased after the two-month intensive phase. Patients will also feel a lot better from the TB being treated. But in some patients antiretroviral therapy needs to start earlier with careful monitoring for IRIS.”
“It’s actually gotten a lot easier to treat the two diseases simultaneously,” Dr. Munsiff continued. “When NNRTIs and PIs first became available in the mid-nineties, there were little data on use of efavirenz and rifampin. There was almost no regimen that was recommended with rifampin. But now there are great antiretroviral regimens that can be given with rifampin as part of a multidrug anti-TB regimen. And outcomes with rifampin and rifabutin are essentially the same.”
| Other Treatment Issues to Consider | Top of page |
Immune Reconstitution Inflammatory Syndrome
The term paradoxical reaction, coined in the pre-AIDS era, describes the development of new manifestations of TB or worsening of existing signs and symptoms of TB in patients on appropriate anti-TB therapy. It is thought to be due to a reversal of a TB-induced state of anergy by effective chemotherapy. IRIS, a term coined in the AIDS era, broadly encompasses a range of inflammatory manifestations temporally related to HAART initiation, resulting either in unmasking a previously occult opportunistic pathogen or in provoking an intensified inflammatory reaction to a concurrent opportunistic pathogen (Manosuthi, 2006). Presumably HAART-induced partial immune restoration permits a heightened inflammatory response. This theory is strengthened by the observation that patients with HIV-associated TB who are started on antiretroviral regimens seem to be at a particularly increased risk of developing this syndrome (Shelburne, 2005). Thus the timing of HAART initiation in patients with TB is further complicated by the potential for a paradoxical or IRIS reaction.
The frequency of IRIS reaction varies from 11% to 45% and occurs more often in patients with lower CD4+ counts, extra-pulmonary disease, disseminated disease, and with a shorter interval from TB diagnosis to antiretroviral initiation (Narita, 1998; Fishman, 2000; Wendel, 2001Navas, 2002; Olalla, 2002; Breton, 2004; Shelburne, 2005; Manosuthi, 2006). Temporally, paradoxical reaction occurs within a few weeks of starting antiretroviral treatment and coincides most closely with viral load decline. The frequency of IRIS was least (13%) in a cohort in whom HAART initiation was delayed until at least five weeks after TB treatment. The frequency of IRIS was similarly low (11%) in an outpatient cohort in which only a minority of patients commenced antiviral therapy concurrent with or after TB, while the rest of the cohort was already on antiretroviral therapy prior to the TB diagnosis.
Paradoxical worsening can manifest in a wide variety of sites, including cervical or mediastinal lymphadenopathy, worsening infiltrates on chest radiograph, or enlarging CNS lesions; fever may or may not be present (Fishman, 2000). The course of paradoxical worsening is often unpredictable; it can be brief or prolonged, with multiple recurrences and exacerbations. These reactions typically occur within a few weeks of starting antiretroviral therapy. The diagnosis of paradoxical reactions remains a diagnosis of exclusion. Diagnosis relies on negative culture of clinical samples, decrease in HIV viral load, and lack of other etiologies, such as relapse of infection, poor adherence to treatment, drug adverse effects, worsening TB due to drug resistance, or other infections.
Treatment of paradoxical reactions is not well established. Mild and moderate reactions can be managed by reassuring the patients or by use of nonsteroidal anti-inflammatory agents. Repeated aspirations for decompression of lymph nodes or other extrapulmonary sites have been used to avoid surgical drainage. Some have advocated the use of corticosteroids or discontinuation of antiretroviral therapy for severe cases—those cases that have lymphadenopathy that may compromise respiration and swallowing, or the development of central nervous system mass lesions. Prednisone at doses ranging from 20 mg to 50 mg daily tapered over as little as two weeks has been used. The use of corticosteroids for these short periods of time does not seem to adversely affect the outcome of the TB treatment. Data on the use of corticosteroids for the treatment of paradoxical reactions in HIV-associated TB are limited; their use should therefore be reserved for severe cases.
Acquired Rifampin Resistance
Another TB treatment issue that is rare, but associated with HIV-infected TB patients, is rifampin resistance, which can develop in patients while on effective treatment. Unlike multidrug-resistant MDR TB, acquired rifampin resistance (ARR) is not associated with isoniazid, demonstrating a more rare resistance pattern. Currently, very little is known about how this is related to HIV infection. One recently published retrospective study that reviewed several years of data from NYC found that HIV-infected patients were at a much higher risk of developing ARR (Li, 2005). Patients with CD4+ counts less than 100 cells/mm3 had an additional elevated risk of ARR and relapse. Intermittent rifampin therapy significantly increased the risk of ARR and relapse when intermittent dosing was begun during the intensive phase. These findings support current CDC recommendations (CDC, 1998) against the use of intermittent regimens during the intensive phase of treatment for patients with TB and advanced HIV infection, and they also support the hypothesis that, for HIV-infected patients with TB, the critical time for development of resistance to rifampin may be in the first 2 months of treatment.
| Conclusion | Top of page |
In conclusion, Dr. Munsiff re-emphasized that TB and HIV are global concerns. “TB among HIV-infected people in New York City has decreased markedly in the past decade. But the problem is increasing worldwide and we need to be aware. There are many immigrants in New York City from high HIV/TB incidence areas. So you need to be aware and think about it when you see patients, especially those from sub-Saharan Africa. In addition, given the large number of HIV-infected people in NYC who live in congregate settings, the potential for TB outbreaks still remains high.”
While the number of TB cases has been decreasing since 1992, the proportion of TB among HIV-infected people remains steady, and it is necessary to know how to diagnose and treat these patients. It is important to be aware that NAA testing can be a helpful tool to identify TB early; TB and antiretroviral drug interactions must be considered when treating both TB and HIV; and new challenges such as IRIS and ARR can complicate treatment. Not only is treatment of TB and HIV critical, but prevention is a lifesaving intervention. If you are managing HIV-infected patients with LTBI, Dr. Munsiff urged, it is essential that they complete their course of treatment.
The most recent NYC DOHMH guidelines, “Antiretroviral Drugs and the Treatment of Tuberculosis” can be found at: http://www.nyc.gov/html/doh/downloads/pdf/tb/tbanti.pdf.
| References | Top of page |
