With the continued widespread use of combination antiretroviral therapy, the incidence of various neurological complications remains low. However, some complications continue to have a serious impact on the lives of HIV-infected patients. The diagnosis of these neurological complications has become even more complex in recent years. Adverse events, stemming from the long-term use of antiretroviral therapy, can lead to neurological complications. And as HIV-positive people continue to live longer because of antiretroviral therapy, the risk of neurological complications stemming from comorbidities increases.
In an effort to debunk modern-day myths surrounding some of the most common neurological complications in the setting of HIV, most notably HIV-associated dementia, neurological opportunistic infections, neoplasms and peripheral neuropathy, PRN brought Dr. Justin McArthur back to the podium to address this important topic.
| Neuromyth No. 1: HIV-Associated Dementia has Disappeared. | Top of page |
On the contrary. Prior to the widespread availability and use of combination antiretroviral therapy, the annual incidence of HIV-associated dementia (HAD) after a diagnosis of AIDS was 7%, with the cumulative risk of developing HIV dementia ranging from 5% to 20%. Today, the incidence of HAD is lower in HIV-infected people, but the prevalence has actually increased. “Since 1994,” Dr. McArthur pointed out, “we’ve essentially seen a doubling of the prevalence of HIV-associated dementia. At the Johns Hopkins HIV clinic, we had approximately 600 cases of HIV dementia in 1994. In 2004, we had approximately 1,400 HIV-infected patients with dementia. Fewer people are dying and we’re seeing people with HIV-related dementia living longer, which contributes to the increasing prevalence.” What’s more, data from the Johns Hopkins HIV clinic suggest that the incidence of HIV dementia has been increasing since 2003.
Dr. McArthur was quick to point out that the dementia being seen in HIV-infected patients today is typically less severe than the dementia seen in the pre-combination therapy era. “We don’t see the really severe dementia that we used to see 15 years ago,” he remarked. “Infectious disease doctors ask: ‘so what’s the problem with a little cognitive impairment?’ But it’s been conclusively shown that the impact of HIV-associated dementia, or even the milder forms ofcognitive impairment, can have a substantial effect on survival, with about a threefold increased risk of death.”
Along with the decreased survival associated with HAD, there are functional issues to be concerned with as well. Dementia can affect driving ability, work performance, and medication adherence. It can also negatively affect compliance with medical appointments, possibly render individuals more susceptible to drugs of abuse, and can impair judgment with respect to safer sex and other HIV-prevention practices. “Clearly, there are a number of diverse aspects to cognitive impairment which can affect daily functioning,” Dr. McArthur added.
A Case Report
To best frame his overview of the complexities of HAD in HIV, Dr. McArthur presented an interesting case report. The case involved a 40-year-old woman with advanced HIV infection who was referred to the Johns Hopkins neurology department because of memory complaints and mental slowing that were interfering with her everyday functioning. “She had to quit work,” Dr. McArthur explained. “She could no longer take care of her medications and had to be supervised on a 24-hour basis because she tended to wander out of the house. She had been drinking heavily until October of 2004 and had a history of DTs [delirium tremens]. Her thiamine level was normal at the time we first saw her. She didn’t have a recent history of Wernicke’s encephalopathy or DTs.”
As for her HIV/medical history, she had spent most of 2004 off of antiretrovirals and then began a combination of lopinavir/ritonavir (Kaletra), zidovudine/lamivudine (Combivir), and tenofovir (Viread). She had a CD4+ count of 42 cells/mm3 and a viral load of 600,000 copies/mL prior to beginning her most recent regimen. She had a history of several HIV- and non-HIV-related illnesses and was taking warfarin (Coumadin) at the time of her neurology consult for bilateral pulmonary embolism.
Brain MRI images from her first study, in early December 2004 when she resumed antiretroviral therapy, yielded subtle white matter hyperintensity around the ventricles (see Figure 1). “In the deep white matter, there was bilateral and fairly symmetrical white matter hyperintensity,” Dr. McArthur explained. “Three months later, after three months of potent antiretroviral therapy, the white matter hyperintensity had extended and became more prominent. This occurred while she was on antiretrovirals.”
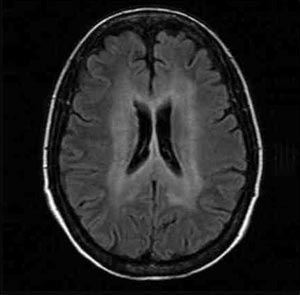
Figure 1. HIV-Associated Dementia
A 40-year-old woman with advanced HIV infection who was referred to the Johns’ Hopkins neurology department because of memory complaints and mental slowing that were interfering with her everyday functioning. She had a history of heavy alcohol use and delirium tremens. She had spent most of 2004 off of antiretrovirals. Brain MRI images from her first study, in early December 2004, yielded bilateral and fairly symmetrical white matter hyperintensity around the ventricles. Three months later, after resuming antiretroviral therapy, the white matter hyperintensity had extended and became more prominent (not shown). Two diagnoses were mande: HIV-associated dementia and severe amnestic syndrome related to Korsakoff’s syndrome, a condition that primarily effects chronic alcoholics.
Source: Justin McArthur, MD
At the time of her second MRI, the patient had a 2 log10 copies/mL drop in HIV-RNA, along with an increase in her CD4+ count to 350 cells/mm3. “There had been some stabilization in her memory, in that she wasn’t getting worse, but she was still severely amnestic and non-functional,” Dr. McArthur said. “We decided to conduct a series of tests, to see what was going on.”
Dr. McArthur’s group first conducted some brief cognitive testing, using a simple three-minute series of tests called the Modified HIV Dementia Scale. The first test is designed to assess memory-registration, whereby the evaluator says four words (e.g., dog, hat, green, peach) and requests that the word be repeated by the patient. The second test is an evaluation of motor speed, in which the patient writes the alphabet in upper-case letters horizontally across the page. Depending on the length of time it takes to complete the alphabet, a specific number of points are allotted (<21 sec. = 6 pts.; 21.1 to 24 sec. = 5 pts.; 24.1 to 27 sec. = 4 pts.; 27.1 to 30 sec. = 3 pts.; 30.1 to 33 sec. = 2 pts.; 33.1 to 36 sec = 1 pts.; > 36 sec. = 0 pts.). The third test evaluates memory-recall, in which the patient is asked to recall the four words used in the first test. One point is awarded for each correct word (maximum total of four points). The last test evaluates construction, in which the patient is asked to copy a 12-line, three-dimensional illustration of a cube. If the illustration is completed successfully in less than 25 seconds, two points are awarded; less than 35 seconds, one point is awarded; and more than 35 seconds, zero points are awarded. “The cutoff for a possible HIV dementia diagnosis is 7.5 out of 12. The patient we’re talking about here had a score of one out of 12. In other words, she was severely impaired.”
Dr. McArthur’s group also performed a lumbar puncture to allow for evaluation of the patient’s cerebrospinal fluid. PCR testing concluded that her CSF viral load was approximately 250,000 copies/mL, compared to the 2,500 copies/mL of HIV RNA in her peripheral blood after three months of treatment. “Essentially,” Dr. McArthur said, “she had a 2 log10 copies/mL discrepancy between her CSF compartment and plasma compartment.” Genotypic testing of HIV in her CSF revealed no major reverse transcriptase or protease mutations conferring drug resistance. “This patient was clearly becoming immunologically stabilized while on antiretroviral therapy,” Dr. McArthur commented. “However, we weren’t seeing much, if any, control of HIV replication in the CSF compartment.”
Might the metabolic syndrome frequently seen in HIV-infected patients receiving combination antiretroviral therapy be to blame? “Metabolic complications of treatment can be associated with early or accelerated cerebrovascular disease, leading to either small- or large-vessel strokes,” he explained. “This was not the case in this patient. The radiologic images did not show any acute or subacute infarcts. Nonetheless, it’s always a possibility to consider with HIV dementia.”
Another possibility to consider is immune reconstitution syndrome, an unusual presentation of opportunistic infectious diseases in patients responding to antiretroviral therapy. In one series briefly described by Dr. McArthur, 22% of patients experienced immune reconstitution illnesses, usually within three to six months of starting treatment. In the central nervous system, immune reconstitution may result in the development or enlargement of cerebral space-occupying lesions after treatment for cerebral cryptococcosis or toxoplasmosis. There have also been reports of PML activation with increased inflammation. “This, however, was not the case with the patient we’re discussing here,” he confided. “But it is a possible cause of neurologic decline in some HIV-infected patients starting antiretroviral therapy with low pre-treatment CD4+ cell counts.”
“CNS escape” is another, albeit rare, situation that can contribute to HIV dementia in patients receiving antiretroviral therapy. According to Dr. McArthur, it occurs primarily in individuals who have been on combination antiretroviral therapy for a period of time and presents as an acute neurological syndrome, usually with an encephalopathy and sometimes with seizures. “It’s different and distinct from the case we’re discussing here, where there was a slowly progressing syndrome with amnesia and memory disturbances as a major feature,” he said. CNS escape is characterized by the discrepancy between low or undetectable HIV-RNA in peripheral blood and high HIV-RNA in CSF, presumably reflecting uncontrolled HIV replication within the CNS. “The differential includes other opportunistic processes, including CMV, herpes simplex encephalitis, as well as immune restoration syndrome,” he added. “It’s usually caused by incomplete adherence to medication regimens, leading to less drug penetrating the CNS compartment.”
Diagnosis of HIV Dementia
The American Academy of Neurology first developed its definitional criteria for HIV dementia in 1991—criteria that are still widely used today. HIV dementia involves a three-part diagnosis: cognitive deficits, functional deficits, and either neurological or psychological deficits (see Table 1). “Probably the most important thing to remember is that HIV dementia is not a diagnosis of exclusion only,” Dr. McArthur explained. “However, one should always think about the complications that can mimic HIV-associated dementia, such as those we looked for in our case patient.
| Table 1. HIV-Associated Dementia Complex | |
| Criteria for 1 and 2 must be met: | And Must meet either 1 or 2 of the following |
|
|
| Source: The American Academy of Neurology | |
The natural history of HIV dementia has changed considerably since combination antiretroviral therapy became the mainstay treatment. In years past, patients with HIV dementia often progressed rapidly, with a median survival of approximately five months. Clinical features of HIV dementia, primarily subcortical in nature, included apathy and severe psychomotor slowing, memory loss, poor insight, gait and motor impairments, hyper-reflexia, hypertonia, and are typically progressive. Today, median survival has been extended to almost four years (Dore, 2003). Clinical features of HIV dementia, which involve mixed cortical and subcortical features, are of a milder phenotype, with frequent transitions and reversals.
A number of laboratory markers have been evaluated in studies, some of which are believed to be associative (demonstrated to have a cross-sectional relationship) and some of which are predictive. “At this point in time, there are no laboratory markers that can be employed in clinical practice to identify individuals who are at risk for HIV-associated dementia,” Dr. McArthur cautioned. “However, there are markers under investigation.
HIV-RNA levels in the CSF have been evaluated as associative and predictive markers. “In the past, viral load in the CSF correlated well with the severity of neurological deficits,” he explained. “However, with the use of combination antiretroviral therapy, recent cohort-based studies have concluded that HIV-RNA levels in CSF cannot distinguish between normal neurological function and minor cognitive impairment related to HIV dementia. So, unfortunately, HIV-RNA in CSF isn’t really a diagnostic tool or a discriminatory tool at this point. However, when one encounters a patient in this day and age who has a mix of both neurological and psychiatric manifestations, higher levels of CSF HIV-RNA tend to indicate more of an organic neurological process than a psychiatric or psychological state.” Dr. McArthur also explained that for clinicians treating patients with established HIV dementia, working to establish better virologic control, monitoring CSF HIV-RNA levels may be useful in terms of gauging whether or not an antiretroviral regimen is working.
Back to the Case Report
“This remains a somewhat unusual case,” Dr. McArthur said in returning to his 40-year-old female patient with memory complaints and mental slowing. “She clearly suffered from features of HIV-associated dementia. She had well controlled plasma HIV-RNA, but uncontrolled CSF, and presumably CNS, HIV replication. And while she was achieving systemic virologic and immunologic control, the white-matter hyperintensities were worsening, although they’re not in the pattern that we typically see with immune restoration syndrome.“
Dr. McArthur’s group ended up making two diagnoses: HIV-associated dementia and severe amnestic syndrome, “which we now believe is probably on the basis of Korsakoff’s syndrome. “Korsakoff’s syndrome is a condition that primarily affects chronic alcoholics. It is not due to the direct effects of alcohol or to other severe nutritional deficiencies that are associated with chronic alcoholism, and is seen specifically in association with vitamin B1 deficiency. The syndrome is characterized by a severe memory defect, especially for recent events, for which the patient compensates by confabulation—the reciting of imaginary experiences. “That, in fact, is what we think this woman is suffering from at this point.”
Antiretroviral Therapy for HIV-Associated Dementia
To achieve greater control of the viral replication in the woman’s CSF, a decision was made to intensify her antiretroviral drug regimen with Ziagen (abacavir). The rationale for considering the use of abacavir included its previously demonstrated penetration into CSF and its activity in macrophages, HIV's principal target cell within the brain. In a recent phase III clinical trial, 105 HIV-positive patients with dementia were randomized to add high-dose abacavir (600 mg BID) or placebo onto stable background antiretroviral regimen (Brew, in press). Overall, both groups showed improvements in neuropsychological performance on standardized tests, with a trend favoring abacavir. The more severely impaired group on abacavir showed a greater improvement than did placebo recipients. By week 12, suppression of plasma viral load to less than 400 copies/mL was observed in 46% of those on abacavir but only 13% of the placebo group. The CSF virological response also favored abacavir with a decrease of 0.64 log10 copies/mL during the study, compared with the placebo group showing a CSF viral load decrease of 0.25 log10 copies/mL. “On the basis of these results, although they’re not a Grand Slam result, we intensified this woman’s antiretroviral regimen with high-dose abacavir.”
Does CNS penetration matter when it comes to putting together an antiretroviral regimen? The data, Dr. McArthur explained, are decidedly mixed. In one evaluation of patients participating in the Multicenter AIDS Cohort Study (MACS), a team of investigators—which included Dr. McArthur—evaluated whether combination antiretroviral therapy involving multiple CSF-penetrating drugs resulted in greater improvement in HIV-associated psychomotor slowing than antiretroviral regimens containing only one CSF-penetrating drug (Sacktor, 2001). Both groups had improvements in their CD4+ cell count and plasma viral loads, as well as two tests of psychomotor speed. Comparing the two groups, however, there were no differences in the mean change for CD4+ cell counts, viral load, or any of the neuropsychological tests. In other words, either multiple or single CSF-penetrating antiretroviral regimens may be equivalent for treating HIV-associated psychomotor slowing.
Another analysis involved a cross-sectional survey of 97 HIV-positive individuals in Australia (Cysique, 2004). The patients were analyzed according to whether their regimen contained three or more CSF-penetrating antiretrovirals (the neuroHAART group) or not (HAART group). Thirty HIV-negative men matched for age and education were recruited as controls. The neuroHAART and HAART groups did not differ from one another on neuropsychological performance, but both patient groups were impaired compared with controls. The neuroHAART group showed significantly better memory performance, unrelated to plasma viral load, than the HAART group. However, the conclusion was that CSF-penetrating antiretroviral regimens did not yield any direct benefit in patients with advanced HIV infection. Only in neuropsychologically impaired patients was there a benefit in memory function. These data suggest that a threshold of neuropsychological impairment is required for the benefit of CSF-penetrating antiretroviral therapy.
Dr. McArthur stressed that there are some overarching principles of antiretroviral therapy for patients with established HIV dementia. These include maximizing antiretrovirals to suppress HIV replication, which includes the use of genotypic and phenotypic drug-resistance assays to determine resistance patterns. It is also preferable that CNS-penetrating antiretroviral agents be used, including zidovudine (Retrovir), stavudine (Zerit), abacavir, efavirenz (Sustiva), nevirapine (Viramune), indinavir (Crixivan), and lopinavir/ritonavir (Kaletra). Given that there may be adherence issues among patients with HIV dementia, it is best to construct simplified regimens, including QD regimens or supervised therapy. Monitoring neurological status is also important and, if progression is noted, reexamination of HIV RNA levels in the CSF. “These are fairly straightforward and sensible recommendations to follow,” Dr. McArthur said.
Adjunctive Therapies for HIV Dementia
Dr. McArthur explained that there have been a number of placebo-controlled trials focusing on the use of adjunctive therapies—therapies used concomitantly with antiretroviral therapy—for HIV-associated dementia. There are the calcium channel blocker nimodipine; the antioxidants selegiline, OPC14117, and thioctic acid; the PAF antagonist lexipafant; memantine, an NMDA antagonist; the TNF-a antagonist CPI-1189; and the chemokine antagonist peptide-T. “All of these agents are focused, not on suppressing HIV replication, but on blocking some of the downstream effects of HIV infection and the inflammatory response to HIV infection,” Dr. McArthur explained. “Most of the studies of these agents only had a modest effect or, in some cases, a negative effect, perhaps with the exception of the anti-Parkinson’s disease drug selegiline. At this stage, I cannot recommend a particular adjunctive therapy for HIV dementia.”
| Neuromyth No. 2: It’s A Guessing Game with CNS Opportunistic Infections. Imaging is so complex. | Top of page |
The Principles of Neurologic Opportunistic Infections
When it comes to opportunistic infections of the central nervous system, there are a few widely accepted principles. First, they usually, but not always, occur in patients with CD4+ counts below 200 cells/mm3. “However, in patients experiencing an immune restoration syndrome, CD4+ counts may have risen above 200 cells/mm3,” Dr. McArthur cautioned. “What’s most important in determining the risk of OIs within the brain is the nadir CD4+ count.”
A second principle is that multiple CNS opportunistic infections can be present. Approximately 15% of patients with one AIDS-related CNS opportunistic infection have a second CNS OI present. “The first patient with cryptococcal meningitis that I ever treated in 1984 actually died from concurrent cerebral toxoplosmosis. I missed the toxoplasmosis and it’s a lesson I continue to remember.”
Generally speaking, maintenance therapy for a neurological infection is a lifelong requirement, unless the patient has an excellent immunological and virological response to antiretroviral therapy. For most patients, this means a CD4+ count that exceeds 200 cells/mm3 for at least six months.
Another principle is that imaging and clinical presentation can be used to distinguish between the different CNS opportunistic infections. “Increasingly, we’re using PCR-based technologies on CSF,” Dr. McArthur commented. “These are particularly helpful for CMV, progressive multifocal leukoencephalopathy, and primary CNS lymphoma. We don’t have validated assays yet for tuberculosis or toxoplasmosis. And while biopsy is 90% sensitive in terms of making a diagnosis, the morbidity and mortality is high.”
The Predictive Value of Signs and Symptoms
A noteworthy study, published in 1999, set out to determine which neurologic signs and symptoms are predictive of new focal lesions in HIV-infected patients (Rothman, 1999). The study enrolled HIV-infected patients who presented at a large inner-city Baltimore emergency department over an 11-month period. Patients were assessed using a standardized neurologic evaluation to ascertain whether they had developed new or changed neurologic signs or symptoms. Patients with any new or changed neurologic findings had a head CT scan in the emergency department. The associative and predictive values between individual complaints or findings and new focal lesions on head CT scans were assessed.
A total of 110 patients were identified as having new or changed neurologic signs or symptoms and had a head CT scan done. Twenty-seven patients (24%) had focal lesions, of which 19 (18%) were identified as new focal lesions; eight of these (7%) demonstrated a mass effect. Clinical findings most strongly associated with new focal findings were: new pattern headaches (i.e., head pain that had never been felt before), headache lasting longer than three days, new seizures, and depressed or altered orientation. Use of these three findings as a screen for ordering head CT scans, the authors concluded, would have identified 100% of the patients with new focal intracranial lesions.
Clinical Features of Neurologic OIs and Cancers
When it comes to the radiologic features of common neurologic opportunistic diseases, Dr. McArthur explained that contrast-enhancing lesions are usually toxoplasmosis or primary CNS lymphoma. Toxoplasmosis usually presents clinically as an acute neurological syndrome with headaches, seizures, and focal neurological deficits. The lesions are generally small, between one to two centimeters. Toxoplasmosis on CT has smooth, peripheral ring enhancement. There are usually multiple rings, and approximately 85% of patients who present with cerebral toxoplasmosis will be seropositive for Toxoplasma gondii antibodies.
Primary CNS lymphoma is usually associated with slowly progressive encephalopathy or focal deficits. CNS lymphoma lesions are typically large—usually >3 cm—with mass effect and complex enhancement (see Figure 2). “It is not a simple ring,” Dr. McArthur said in describing the radiologic findings. “It’s a complex, heterogeneously enhancing lesion that appears to have nodules in the wall of the lesion.” Confusing matters is the fact that approximately 15% of patients with CNS lymphoma are seropositive for T. gondii antibodies. Approximately 50% of patients with CNS lymphoma will have measurable Epstein-Barr virus (EBV) in the CSF on PCR.
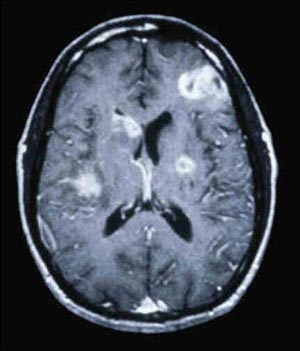
Figure 2. Primary Central Nervous System Lymphoma Primary CNS lymphoma is usually associated with slowly progressive encephalopathy or focal deficits. CNS lymphoma lesions are typically large (usually >3 cm) with mass effect and complex enhancement. Approximately 50% of patients with CNS lymphoma will have measurable Epstein-Barr virus (EBV) in the CSF on PCR.
Source: Justin McArthur, MD
“One can use radiology in many cases to separate, or at least sharpen their focus, when distinguishing between toxoplasmosis and primary CNS lymphoma,” Dr. McArthur said. “A thallium SPECT scan may also be helpful. Thallium is the same isotope that’s used for myocardial imaging. It’s taken up into tumors but not into abscesses. A thallium SPECT scan can show focal uptake of the radiotracer into the lesion and has a sensitivity and specificity of greater than 90%. When you’re not sure whether its toxoplasmosis or primary CNS lymphoma, a thallium SPECT scan can be quite helpful.”
The two non-enhancing lesions seen in AIDS are usually PML or cryptococcal meningitis. With PML, the presentation usually involves subacute progression of focal neurological deficits. PML is not typically associated with headaches, seizures, or fever. Subcortical white matter lesions are the typical radiologic finding. “The lesions are generally hard edged, not fluffy or indistinct as we see with HIV-associated dementia white-matter lesions,” Dr. McArthur explained.
Cryptococcomas usually present as acute meningitis with headaches, encephalopathy, and elevated intracranial pressure. Neck stiffness is usually absent. “Cryptococcomas usually form within the basal ganglia,” he said. “Cryptococcal meningitis does not elicit much of an inflammatory response. The lesions are small and tend to be punched out, almost like lacunar infarcts.”
The Management of PML
Dr. McArthur spent some time discussing the management of PML, which has long been one of the most difficult-to-treat AIDS-related opportunistic infections. First, it is recommended that the use of any immunosuppressive agents be avoided or reduced. “If patients are on steroids or other immunosuppressives, the first thing we do is reduce the dose or discontinue the drug,” he said. Control of HIV replication is another important factor to consider. “A number of studies have found that the most important treatment for PML is to maximize antiretroviral therapy.”
As for adjunctive therapy, topoisomerase inhibitors were found to be too toxic for the treatment of PML. As for cidofovir, experience in Europe has been more positive than in the United States, where it is rarely if ever used for the management of PML. Cytosine arabinoside hasn’t been demonstrated to have any positive effect on survival rates of HIV-infected patients with PML and should not be used. More encouragingly, there have been some positive in vitro data involving the serotonin receptor (5HT2A) blockers cyproheptadine and mirtazapine (Remeron) (Elphick, 2004). “In test tube studies, these agents blocked the growth of the JC virus, the cause of PML,” Dr. McArthur commented. There have also been encouraging data and reports surrounding the use of interferon-alfa. “This has been widely used in a series of uncontrolled studies. In several studies, interferon improved survival and induced some regression of PML lesions.”
| Table 2. The Two-Minute HIV NeuroScreen | |
| Abnormality | Possible Diagnosis |
| Memory loss, slow mentation | Dementia |
| Cauda equina syndrome | CMV radiculitis |
| Leg weakness, sensory level | Myelopathy, epidural abscess |
| Ascending paresis | Guillain-Barre syndrome, lactic acidosis |
| Pain in feet, absent ankle jerks | Sensory neuropathy |
| Seizures, focal deficits | Toxoplasmosis |
| Slowly progressive deficits | Progressive multifocal leukoencephalopathy |
| Cranial neuropathies, intracranial pressure elevation | Cryptococcal meningitis |
| Source: Justin McArthur, MBBS, MPH | |
| Neuromyth No. 3: If It Tingles, It Must be Drug Neuropathy | Top of page |
A Case Report
In introducing his discussion on peripheral neuropathy, Dr. McArthur began with another case report. The patient is a 23-year-old female with HIV infection, and CD4+ count of 534 cells/mm3, and minimal exposure to antiretroviral therapy. She presented with numbness and tingling in the feet associated with ankle and toe weakness. Examination revealed that she had distal weakness, high arches and hammer toes. Ankle reflexes were absent and she had impaired sensation. Comorbidities included hepatitis C virus coinfection and diabetes mellitus. She also had a family history of “nerve disease.”
“A number of things were going on in this patient that could potentially be contributing to the development of sensory neuropathy,” Dr. McArthur remarked. “The diagnosis, we realized, was going to be quite difficult.” Dr. McArthur’s group first considered distal sensory polyneuropathy (DSP). “This is triggered typically by HIV infection,” he commented. “However, the incidence rates of DSP are low in patients who have CD4+ counts above 500 cells/mm3.” Antiretroviral toxic neuropathy (ATN) was also explored as a possibility. “Her exposure to antiretrovirals was very limited, lasting no longer than a few months. It’s highly unlikely that she was dealing with neuropathy stemming from antiretrovirals.” Dr. McArthur’s group also considered entrapment neuropathy which is caused by physical compression or irritation of major nerve trunks and peripheral nerves. Another possibility was hereditary sensorimotor neuropathy, “which is what we believe this woman was experiencing,” he said. “She had Charcot-Marie-Tooth disease,” an inherited neurological disease characterized by a slowly progressive degeneration of the muscles in the foot, lower leg, hand, and forearm, and a mild loss of sensation in the limbs, fingers, and toes. “This reiterates the point that neurological diseases do not always have to be linked to HIV or to the consequences of treatment.”
Neuromuscular Syndromes in HIV
There are several major neuromuscular syndromes seen in HIV disease. First there are the sensory neuropathies, which include DSP and ATN. “Sensory neuropathies have a variable incidence rate,” Dr. McArthur explained. “They’re much more common in individuals who have lower CD4+ cell counts, with ATN occurring in HIV-positive individuals receiving antiretrovirals known to cause neuropathy.” There is also mononeuropathy, which can occur in early HIV disease, but is considered rare and usually only affects one nerve. Later in HIV disease, it is a picture of mononeuritis multiplex, being progressive and usually involves multiple nerves. Frequently, patients with mononeuropathy multiplex are coinfected with HCV. Then there is progressive polyradiculopathy, which presents as a cauda equine syndrome and is usually seen in patients with low CD4+ cell counts and active CMV infection. Inflammatory demyelinating polyneuropathy, manifesting as Guillain-Barre syndrome, is usually a feature of early HIV disease, when the immune system is still relatively intact. There have also been reports of myopathy in HIV-infected individuals, which can manifest as polymyositis early in the course of HIV infection or at any stage of disease in association with antiretroviral use. Rarely, amyotrophic lateral sclerosis has been documented in HIV-positive patients, usually in later stages of the disease. “We don’t know whether this is a consequence of HIV or just a serendipitous finding,” Dr. McArthur commented. Finally, there is the HIV-associated neuromuscular weakness syndrome, which has been documented in patients receiving antiretrovirals associated with mitochondrial toxicity.
Just as there are confounding illnesses that can contribute to neuropsychological problems, there are also confounding illnesses that can contribute to neuromuscular problems in HIV-infected individuals. For starters, there is impaired glucose tolerance, which has become a major problem for HIV-infected individuals, particularly those being treated with protease inhibitors. “In the Hopkins’ service, 11% of our patients receiving antiretroviral therapy have diabetes mellitus, with a total of 20% with impaired glucose tolerance,” Dr. McArthur pointed out. “Increasingly, particularly as these individuals have longer periods of hyperglycemia, we’re going to encounter peripheral neuropathies stemming from frank diabetes mellitus.” Alcohol abuse and hepatitis C are two additional entities that have been linked to the development of sensory neuropathies. Entrapment neuropathies are seen more commonly in patients with underlying HIV-associated neuropathy. Sensory neuropathies are seen in patients ingesting excessive amounts of vitamin B6 ( pyridoxine) which is used by some HIV-infected patients.
“The problem with sensory neuropathies is that they aren’t only about annoying tingling and numbness,” Dr. McArthur said. They can have a profound effect on quality-of-life. They can also greatly limit the choice of antiretroviral regimens, through the process of eliminating and avoiding neurotoxic agents. Sensory neuropathies also contribute to reduced adherence. “I’ve had a number of patients who have reduced their dose or skipped doses altogether, because they’re afraid of worsening neuropathy.” Finally, from a pathogenic standpoint, sensory neuropathies may also be a marker of mitochondrial toxicity.”
Screening and Diagnosis of Sensory Neuropathies
In terms of screening and diagnosing patients with neuropathy, the process begins with a basic question: Does the patient have any pain, numbness, or tingling in the legs? From there, ankle reflexes are checked “in a careful, standardized way.” After the ankle reflexes are checked, vibration threshold in the toes is evaluated. Reduced vibration—that is, on the dorsal surface of the great toe in less than 10 seconds—is indicative of neuropathy. “This initial screening takes a minute or less to do,” Dr. McArthur said. “It can identify neuropathy with a sensitivity of approximately 64%. The specificity is even higher—approximately 91% of patients who pass the preliminary screening aren’t likely suffering from a sensory neuropathy.”
More elaborate testing includes the collection of blood samples to screen for diabetes mellitus and vitamin B12 deficiency. Electrodiagnostic procedures are also possible and, in the case of sensory neuropathies, will yield evidence of decreased nerve conduction velocity or amplitude, as well as EMG evidence of active or chronic partial denervation with reinnervation in distal leg muscles. CSF analyses and nerve biopsies can also be conducted, but are widely considered to be unnecessary in the clinical diagnosis of sensory neuropathies, other than for research purposes.
Management of Sensory Neuropathies
Sensory neuropathies can be difficult to manage, and the primary goal of treatment is to help alleviate symptoms. For starters, there is often a significant benefit in stopping or switching an offending HIV/AIDS drug, but this must be balanced against the antiviral benefit that the drug is providing to the patient.
Mild neuropathic pain can sometimes be treated using non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen; moderate to severe cases may respond to tricyclic antidepressants (e.g., amitriptyline or nortriptyline), anticonvulsants (e.g., phenytoin, oxcarbazapine, and gabapentin), or narcotic analgesics (e.g., methadone or fentanyl transdermal patches). Yet, none of these treatments has proved to be effective in clinical trials.
Lamotrigine (Lamictal), a novel anticonvulsant, has shown to be effective in two placebo-controlled trials. “These trials have shown a very gratifying effect on HIV-associated sensory neuropathy,” Dr. McArthur pointed out. “It’s metabolized through glucoronidation, so it doesn’t interfere with the protease inhibitors. Its major drawback is rash which occurs in approximately 5% of patients.” Another anticonvulsant being explored for the treatment of sensory neuropathies is topiramate (Topamax).
Finally, there is duloxetine (Cymbalta), an antidepressant that has been licensed for the treatment of diabetic neuropathy. Duloxetine functions as a serotonin and norepinephrine reuptake inhibitor. “It’s well tolerated by most patients, with the exception of some nauea,” Dr. McArthur said. “Plus, it’s a once-a-day drug.”
| References | Top of page |
